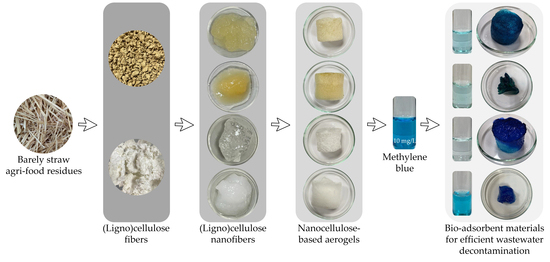
Nanostructured Cellulose-Based Aerogels: Influence of Chemical/Mechanical Cascade Processes on Quality Index for Benchmarking Dye Pollutant Adsorbents in Wastewater Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Barley Straw Cellulose Fibers Characterization
2.2. Quality Index of (L)CNFs and Chemical Characterization
2.3. (L)CNF-Based Aerogels Characterization
2.4. Adsorption Behavior of (L)CNF-Based Aerogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Cellulose Fibers’ Isolation and Characterization
4.3. Cellulose Nanofibers’ Isolation
4.4. Quality Index Determination and Cellulose Nanofibers’ Characterization
4.5. (L)CNF-Based Aerogels Preparation and Characterization
4.6. Dye Removal Efficiency of (L)CNF-Based Aerogels
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- William, R.; Morin, K.H. Transforming Our World: The 2030 Agenda for Sustainable Development. In A New Era in Global Health: Nursing and the United Nations 2030 Agenda for Sustainable Development; Springer Publishing Company: New York, NY, USA, 2018; pp. 109–126. [Google Scholar]
- Alzate, C.A.C.; Ortiz-Sanchez, M.; Solarte-Toro, J.C. Design strategy of food residues biorefineries based on multifeedstocks analysis for increasing sustainability of value chains. Biochem. Eng. J. 2023, 194, 108857. [Google Scholar] [CrossRef]
- Leyva-López, N.; Lizárraga-Velázquez, C.E.; Hernández, C.; Sánchez-Gutiérrez, E.Y. Exploitation of agro-industrial waste as potential source of bioactive compounds for aquaculture. Foods 2020, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, A.; Donsì, F. Impact of High-Pressure Homogenization on Enhancing the Extractability of Phytochemicals from Agri-Food Residues. Molecules 2023, 28, 5657. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, A.; Olivieri, F.; Castaldo, R.; Gentile, G.; Donsì, F. Cellulose Isolation from Tomato Pomace: Part II—Integrating High-Pressure Homogenization in a Cascade Hydrolysis Process for the Recovery of Nanostructured Cellulose and Bioactive Molecules. Foods 2023, 12, 3221. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Rincón, E.; Morcillo-Martín, R.; Rabasco-Vílchez, L.; Rodríguez, A. Orange peel waste biorefinery in multi-component cascade approach: Polyphenolic compounds and nanocellulose for food packaging. Ind. Crops Prod. 2022, 187, 115413. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M.W.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Pirozzi, A.; Ferrari, G.; Donsì, F. Cellulose Isolation from Tomato Pomace Pretreated by High-Pressure Homogenization. Foods 2022, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, N.; Umesh, M.; Selvaraj, M.; Al-Shehri, B.M.; Chakraborty, P.; Duhan, L.; Sharma, S.; Pasrija, R.; Awasthi, M.K.; et al. Emerging challenges for the agro-industrial food waste utilization: A review on food waste biorefinery. Bioresour. Technol. 2022, 362, 127790. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, S.; Ma, X.; Wang, S. Characterization and acetylation behavior of bamboo pulp. Wood Sci. Technol. 2008, 42, 621–632. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 1. [Google Scholar] [CrossRef] [PubMed]
- Das, A.M.; Hazarika, M.P.; Goswami, M.; Yadav, A.; Khound, P. Extraction of cellulose from agricultural waste using Montmorillonite K-10/LiOH and its conversion to renewable energy: Biofuel by using Myrothecium gramineum. Carbohydr. Polym. 2016, 141, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Rengel, Z. The Role of Crop Residues in Improving Soil Fertility. In Nutrient Cycling in Terrestrial Ecosystems; Marschner, P., Rengel, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 183–214. [Google Scholar]
- Rodriguez-Gomez, D.; Lehmann, L.; Schultz-Jensen, N.; Bjerre, A.B.; Hobley, T.J. Examining the potential of plasma-assisted pretreated wheat straw for enzyme production by Trichoderma reesei. Appl. Biochem. Biotechnol. 2012, 166, 2051–2063. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.; Jawaid, M. Recent advances in nanocellulose-based polymer nanocomposites. In Cellulose-Reinforced Nanofibre Composites: Production, Properties and Applications; Woodhead Publishing: Cambrige, UK, 2017; pp. 89–112. ISBN 9780081009659. [Google Scholar]
- Pirozzi, A.; Ferrari, G.; Donsì, F. The use of nanocellulose in edible coatings for the preservation of perishable fruits and vegetables. Coatings 2021, 11, 990. [Google Scholar] [CrossRef]
- Ma, H.; Hsiao, B.S. Nanocellulose Extracted from Defoliation of Ginkgo Leaves. MRS Adv. 2018, 3, 2077–2088. [Google Scholar] [CrossRef]
- Pirozzi, A.; Capuano, R.; Avolio, R.; Gentile, G.; Ferrari, G.; Donsì, F. O/W pickering emulsions stabilized with cellulose nanofibrils produced through different mechanical treatments. Foods 2021, 10, 1886. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; Jiang, F. Sustainable isolation of nanocellulose from cellulose and lignocellulosic feedstocks: Recent progress and perspectives. Carbohydr. Polym. 2021, 267, 118188. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose nanomaterials-binding properties and applications: A review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef] [PubMed]
- Desmaisons, J.; Boutonnet, E.; Rueff, M.; Dufresne, A.; Bras, J. A new quality index for benchmarking of different cellulose nanofibrils. Carbohydr. Polym. 2017, 174, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Buchtová, N.; Budtova, T. Cellulose aero-, cryo- and xerogels: Towards understanding of morphology control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent Progress on Nanocellulose Aerogels: Preparation, Modification, Composite Fabrication, Applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef]
- Long, L.Y.; Weng, Y.X.; Wang, Y.Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef]
- Kim, D.Y.; Han, G.T.; Shin, H.S. Adsorption of polycyclic aromatic hydrocarbons (PAHs) by cellulosic aerogels during smoked pork sausage manufacture. Food Control 2021, 124, 107878. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Zhang, S.; Zhang, Y.; Yuan, Z.; Li, H.; Jiang, J. Microstructure and performance characterization of thermal-insulating and water-resistive aerogel incorporated cement-based materials. Cem. Concr. Compos. 2022, 130, 104535. [Google Scholar] [CrossRef]
- Wong, J.C.H.; Kaymak, H.; Brunner, S.; Koebel, M.M. Mechanical properties of monolithic silica aerogels made from polyethoxydisiloxanes. Microporous Mesoporous Mater. 2014, 183, 23–29. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem.-Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- Ratynskaia, S.; Bergsker, H.; Emmoth, B.; Litnovsky, A.; Kreter, A.; Philipps, V. Capture by aerogel—Characterization of mobile dust in tokamak scrape-off layer plasmas. Nucl. Fusion 2009, 49, 122001. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Nguyen, S.T.; Fan, Z.; Duong, H.M. Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chem. Eng. J. 2015, 270, 168–175. [Google Scholar] [CrossRef]
- Morcillo-Martín, R.; Espinosa, E.; Rabasco-Vílchez, L.; Sanchez, L.M.; de Haro, J.; Rodríguez, A. Cellulose Nanofiber-Based Aerogels from Wheat Straw: Influence of Surface Load and Lignin Content on Their Properties and Dye Removal Capacity. Biomolecules 2022, 12, 232. [Google Scholar] [CrossRef]
- Carpenter, A.W.; De Lannoy, C.F.; Wiesner, M.R. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef]
- Alsukaibi, A.K.D. Various Approaches for the Detoxification of Toxic Dyes in Wastewater. Processes 2022, 10, 1968. [Google Scholar] [CrossRef]
- Fito, J.; Abrham, S.; Angassa, K. Adsorption of Methylene Blue from Textile Industrial Wastewater onto Activated Carbon of Parthenium hysterophorus. Int. J. Environ. Res. 2020, 14, 501–511. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Chcn, G.; Pan, J.; Han, B.; Yan, H. Adsorption of methylene blue on montmorillonite. J. Dispers. Sci. Technol. 1999, 20, 1179–1187. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye using activated carbon prepared from oil palm shell: Batch and fixed bed studies. Desalination 2008, 225, 13–28. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Al-Ani, F.H.; Al-Shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of dyes using graphene oxide (Go) mixed matrix membranes. Membranes 2020, 10, 366. [Google Scholar] [CrossRef]
- Shi, B.; Li, G.; Wang, D.; Feng, C.; Tang, H. Removal of direct dyes by coagulation: The performance of preformed polymeric aluminum species. J. Hazard. Mater. 2007, 143, 567–574. [Google Scholar] [CrossRef]
- Rehman, F.; Sayed, M.; Khan, J.A.; Khan, H.M. Removal of crystal violet dye from aqueous solution by gamma irradiation. J. Chil. Chem. Soc. 2017, 62, 3359–3364. [Google Scholar] [CrossRef]
- Suzuki, N.; Okazaki, A.; Takagi, K.; Serizawa, I.; Hirami, Y.; Noguchi, H.; Pitchaimuthu, S.; Terashima, C.; Suzuki, T.; Ishida, N.; et al. Complete decomposition of sulfamethoxazole during an advanced oxidation process in a simple water treatment system. Chemosphere 2022, 287, 132029. [Google Scholar] [CrossRef]
- Gorito, A.M.; Pesqueira, J.F.J.R.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Nunes, O.C.; Almeida, C.M.R.; Silva, A.M.T. Ozone-based water treatment (O3, O3/UV, O3/H2O2) for removal of organic micropollutants, bacteria inactivation and regrowth prevention. J. Environ. Chem. Eng. 2021, 9, 10–14. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Perumalsamy, M.; Janardhana Prabhu, H. Decolourization potential of white-rot fungus Phanerochaete chrysosporium on synthetic dye bath effluent containing Amido black 10B. J. Saudi Chem. Soc. 2014, 18, 845–853. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Gupta, V.K.; Bhatnagar, A. Suhas Utilization of industrial waste products as adsorbents for the removal of dyes. J. Hazard. Mater. 2003, 101, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Chen, Y.; Zhao, X.; Tan, F.; Wang, Y.; Cao, Y.; Cai, W. Effective removal of cation dyes from aqueous solution using robust cellulose sponge. J. Saudi Chem. Soc. 2020, 24, 915–924. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Xu, X.; Miao, C.; He, T.; Jiang, B.; Wu, W. Preparation of Bio-Based Aerogel and Its Adsorption Properties for Organic Dyes. Gels 2022, 8, 755. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Espinosa, E.; Tarrés, Q.; Delgado-Aguilar, M.; González, I.; Mutjé, P.; Rodríguez, A. Suitability of wheat straw semichemical pulp for the fabrication of lignocellulosic nanofibres and their application to papermaking slurries. Cellulose 2016, 23, 837–852. [Google Scholar] [CrossRef]
- Tang, K.; Hao, X.; Wei, Q.; Zhou, X. Effects of Lignin Chemistry on Cellulose Extraction Performance Towards Crop Straw/Stalk. Chiang Mai J. Sci. 2020, 47, 1204–1215. [Google Scholar]
- Hubbe, M.A. Water vapor barrier properties of coated and filled microfibrillated cellulose composite films. BioResources 2011, 6, 4370–4388. [Google Scholar] [CrossRef]
- Chaker, A.; Alila, S.; Mutje, P.; Rei, M.; Sami, V. Key role of the hemicellulose content and the cell morphology on the nanofibrillation effectiveness of cellulose pulps. Cellulose 2013, 20, 2863–2875. [Google Scholar] [CrossRef]
- Soon, L.; Chiang, L. Influence of different extraction solvents on lipophilic extractives of Acacia hybrid in different wood portions. Asian J. Appl. Sci. 2012, 5, 107–116. [Google Scholar] [CrossRef]
- Ververis, C.; Georghiou, K.; Danielidis, D.; Hatzinikolaou, D.G.; Santas, P.; Santa, R.; Corleti, V. Cellulose, hemicelluloses, lignin and ash content of some organic materials and their suitability for use as paper pulp supplements. Bioresour. Technol. 2007, 98, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Calculation of Mark–Houwink–Sakurada (MHS) equation viscometric constants for chitosan in any solvent–temperature system using experimental reported viscometric constants data. Carbohydr. Polym. 2007, 68, 477–488. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, C.W.; Han, S.Y.; Kwon, G.J.; Kim, N.H.; Lee, S.H. Effects of pH on nanofibrillation of TEMPO-oxidized paper mulberry bast fibers. Polymers 2019, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Shibata, I.; Isogai, A. Depolymerization of cellouronic acid during TEMPO-mediated oxidation. Cellulose 2003, 10, 151–158. [Google Scholar] [CrossRef]
- Espinosa, E.; Sánchez, R.; González, Z.; Domínguez-Robles, J.; Ferrari, B.; Rodríguez, A. Rapidly growing vegetables as new sources for lignocellulose nanofibre isolation: Physicochemical, thermal and rheological characterisation. Carbohydr. Polym. 2017, 175, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishiyama, Y.; Putaux, J.; Vignon, M.; Isogai, A. Homogeneous Suspensions of Individualized Microfibrils from TEMPO-Catalyzed Oxidation of Native Cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Osorio, D.A.; Cranston, E.D. Optimization of cellulose nanocrystal length and surface charge density through phosphoric acid hydrolysis. Philos. Trans. A Math. Phys. Eng. Sci. 2018, 376, 20170041. [Google Scholar] [CrossRef]
- Aguado, R.; Tarr, Q.; Mutj, P.; Angels, M.; Delgado-aguilar, M. Industrial Crops & Products Non-covalently cationized nanocellulose from hemp: Kinetics, key properties, and paper strengthening. Ind. Crop. Prod. 2022, 188, 115582. [Google Scholar] [CrossRef]
- Thi Thanh Hop, T.; Thi Mai, D.; Duc Cong, T.; Thi, Y.; Nhi, T.; Duc Loi, V.; Thi Mai Huong, N.; Trinh Tung, N. A comprehensive study on preparation of nanocellulose from bleached wood pulps by TEMPO-mediated oxidation. Results Chem. 2022, 4, 100540. [Google Scholar] [CrossRef]
- Cui, F.; Li, H.; Chen, C.; Wang, Z.; Liu, X.; Jiang, G.; Cheng, T. Cattail fibers as source of cellulose to prepare a novel type of composite aerogel adsorbent for the removal of enrofloxacin in wastewater. Int. J. Biol. Macromol. 2021, 191, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Li, S.M.; Ma, M.G.; Zhu, J.F.; Sun, R.C. Synthesis and characterization of cellulose-silica composite fiber in ethanol/water mixed solvents. BioResources 2011, 6, 1186–1195. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zhou, Q.; Berglund, L.A. High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC). Compos. Sci. Technol. 2011, 71, 1593–1599. [Google Scholar] [CrossRef]
- Ali, Z.M.; Gibson, L.J. The structure and mechanics of nanofibrillar cellulose foams. Soft Matter 2013, 9, 1580–1588. [Google Scholar] [CrossRef]
- Woignier, T.; Reynes, J.; Hafidi Alaoui, A.; Beurroies, I.; Phalippou, J. Different kinds of structure in aerogels: Relationships with the mechanical properties. J. Non-Cryst. Solids 1998, 241, 45–52. [Google Scholar] [CrossRef]
- Sescousse, R.; Gavillon, R.; Budtova, T. Aerocellulose from cellulose-ionic liquid solutions: Preparation, properties and comparison with cellulose-NaOH and cellulose-NMMO routes. Carbohydr. Polym. 2011, 83, 1766–1774. [Google Scholar] [CrossRef]
- Aguado, R.J.; Mazega, A.; Tarrés, Q.; Delgado-Aguilar, M. The role of electrostatic interactions of anionic and cationic cellulose derivatives for industrial applications: A critical review. Ind. Crops Prod. 2023, 201, 116896. [Google Scholar] [CrossRef]
- Vargas, F.; González, Z.; Sánchez, R.; Jiménez, L.; Rodríguez, A. Cellulosic pulps of cereal straws as raw material for the manufacture of ecological packaging. BioResources 2012, 7, 4161–4170. [Google Scholar] [CrossRef]
- Technical Association of the Pulp & Paper Industry Inc. TAPPI Standards: Regulations and Style Guidelines; Technical Association of the Pulp & Paper Industry Inc.: Peachtree Corners, GA, USA, 2018. [Google Scholar]
- Marx-Figini, M. The acid-catalyzed degradation of cellulose linters in distinct ranges of degree of polymerization. J. Appl. Polym. Sci. 1987, 3, 2097–2105. [Google Scholar] [CrossRef]
- Espinosa, E.; Sánchez, R.; Otero, R.; Domínguez-Robles, J.; Rodríguez, A. A comparative study of the suitability of different cereal straws for lignocellulose nanofibers isolation. Int. J. Biol. Macromol. 2017, 103, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Lindström, T.; Sundström, J. Repeated homogenization, a route for decreasing the energy consumption in the manufacturing process of carboxymethylated nanofibrillated cellulose? Cellulose 2015, 22, 1147–1157. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, F.; Mutjé, P.; Pelach, M.A. Control of retention in paper-making by colloid titration and zeta potential techniques. Wood Sci. Technol. 1998, 32, 145–155. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Liu, R.L.; Liu, Y.; Zhou, X.Y.; Zhang, Z.Q.; Zhang, J.; Dang, F.Q. Biomass-derived highly porous functional carbon fabricated by using a free-standing template for efficient removal of methylene blue. Bioresour. Technol. 2014, 154, 138–147. [Google Scholar] [CrossRef]









| BS | BS-UB | BS-B | ||
|---|---|---|---|---|
| Extractives in water | (%) | 14.60 ± 0.50 c | 4.40 ± 0.05 b | 3.53 ± 0.3 a |
| Extractives in EtOH | (%) | 10.24 ± 0.24 a | 13.91 ± 0.11 c | 12.83 ± 0.50 b |
| Ashes | (%) | 7.38 ± 0.04 c | 1.41 ± 0.01 b | 0.82 ± 0.01 a |
| Lignin | (%) | 11.88 ± 1.45 b | 10.30 ± 0.70 b | 1.09 ± 0.04 a |
| Hemicellulose | (%) | 22.80 ± 0.59 b | 21.48 ± 0.13 a | 21.73 ± 0.16 a |
| α-cellulose | (%) | 34.86 ± 0.33 a | 47.41 ± 0.70 b | 60.66 ± 0.48 c |
| Cellulose extraction yield | (%) | – | 33.41 ± 3.67 b | 26.85 ± 2.75 a |
| LCNF-TO | CNF-TO | LCNF-Mec | CNF-Mec | ||
|---|---|---|---|---|---|
| Nanofibrillation yield | (%) | 61.24 ± 1.00 c | 89.70 ± 0.87 d | 16.08 ± 0.35 a | 26.17 ± 0.45 b |
| Cationic demand | (µeq/g) | 624.80 ± 35.15 b | 732.40 ± 40.05 c | 292.96 ± 21.82 a | 333.49 ± 37.29 c |
| Carboxyl content | (µmol/g) | 431.11 ± 109.03 c | 653.06 ± 41.72 b | 153.32 ± 11.33 a | 157.55 ± 88.60 a |
| ζ-potential | (mV) | –19.13 ± 6.92 b | –63.97 ± 4.41 a | –20.00 ± 0.75 b | –25.77 ± 1.21 b |
| pH | (-) | 7.56 ± 0.22 b | 7.16 ± 0.15 b | 6.48 ± 0.16 a | 6.27 ± 0.35 c |
| Viscosity | (mL/g) | 225.15 ± 23.20 a | 189.69 ± 28.71 a | 518.09 ± 14.36 b | 512.78 ± 23.21 b |
| Polymerization degree | (-) | 536.07 ± 55.23 a | 451.65 ± 68.36 a | 1233.56 ± 34.18 b | 1220.90 ± 55.26 b |
| Turbidity | (NTU) | 56.95 ± 2.47 b | 15.02 ± 3.44 a | 289.50 ± 9.19 d | 189.05 ± 10.39 c |
| Young’s Modulus | (MPa) | 30.08 ± 0.26 c | 37.27 ± 1.24 d | 9.59 ± 1.04 b | 4.86 ± 0.68 a |
| Parameters | Carboxyl Content | Cationic Demand |
|---|---|---|
| y0 | 6.038 | 190.023 |
| a | 7.096 | 6.333 |
| R2 | 0.986 | 0.976 |
| Adj R2 | 0.979 | 0.964 |
| LCNF-TO | CNF-TO | LCNF-Mec | CNF-Mec | ||
|---|---|---|---|---|---|
| Young’s Modulus | (kPa) | 30.46 ± 2.19 c | 33.91 ± 3.92 c | 18.68 ± 2.71 a | 24.21 ± 1.72 b |
| Tensile strength | (kPa) | 2.78 ± 0.32 b | 3.09 ± 0.17 b | 1.81 ± 0.05 a | 1.61 ± 0.18 a |
| Stiffness | (kN/m) | 6.96 ± 0.48 b | 7.23 ± 0.16 b | 4.73 ± 0.85 a | 5.51 ± 0.78 a |
| Apparent density | (kg/m3) | 7.53 ± 0.50 c | 9.08 ± 0.43 d | 5.78 ± 0.40 a | 6.61 ± 0.16 b |
| Porosity | (%) | 99.51 ± 0.03 b | 99.41 ± 0.03 a | 99.62 ± 0.03 d | 99.57 ± 0.01 c |
| Parameters | LCNF-TO | CNF-TO | LCNF-Mec | CNF-Mec |
|---|---|---|---|---|
| y0 | 1.042 ± 0.025 | 0.708 ± 0.042 | 0.857 ± 0.111 | 4.765 ± 0.774 |
| a | 9.250 ± 0.484 | 9.283 ± 0.557 | 9.028 ± 0.661 | 5.231 ± 0.359 |
| b | 0.346 ± 0.004 | 0.043 ± 0.013 | 0.005 ± 0.001 | 0.377 ± 0.013 |
| R2 | 0.999 ± 0.001 | 0.997 ± 0.001 | 0.994 ± 0.004 | 0.947 ± 0.006 |
| Adj R2 | 0.998 ± 0.001 | 0.996 ± 0.001 | 0.992 ± 0.006 | 0.928 ± 0.008 |
| Parameters | Value |
|---|---|
| Qm | 4.677 |
| KL | 19.796 |
| R2 | 0.997 |
| Adj R2 | 0.996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirozzi, A.; Rincón, E.; Espinosa, E.; Donsì, F.; Serrano, L. Nanostructured Cellulose-Based Aerogels: Influence of Chemical/Mechanical Cascade Processes on Quality Index for Benchmarking Dye Pollutant Adsorbents in Wastewater Treatment. Gels 2023, 9, 958. https://doi.org/10.3390/gels9120958
Pirozzi A, Rincón E, Espinosa E, Donsì F, Serrano L. Nanostructured Cellulose-Based Aerogels: Influence of Chemical/Mechanical Cascade Processes on Quality Index for Benchmarking Dye Pollutant Adsorbents in Wastewater Treatment. Gels. 2023; 9(12):958. https://doi.org/10.3390/gels9120958
Chicago/Turabian StylePirozzi, Annachiara, Esther Rincón, Eduardo Espinosa, Francesco Donsì, and Luis Serrano. 2023. "Nanostructured Cellulose-Based Aerogels: Influence of Chemical/Mechanical Cascade Processes on Quality Index for Benchmarking Dye Pollutant Adsorbents in Wastewater Treatment" Gels 9, no. 12: 958. https://doi.org/10.3390/gels9120958
APA StylePirozzi, A., Rincón, E., Espinosa, E., Donsì, F., & Serrano, L. (2023). Nanostructured Cellulose-Based Aerogels: Influence of Chemical/Mechanical Cascade Processes on Quality Index for Benchmarking Dye Pollutant Adsorbents in Wastewater Treatment. Gels, 9(12), 958. https://doi.org/10.3390/gels9120958