Poly(Sulfobetaine Methacrylate-co-Vinyl Pyrrolidone) Hydrogels as Potential Contact Lenses Delivery Systems for Timolol Maleate
Abstract
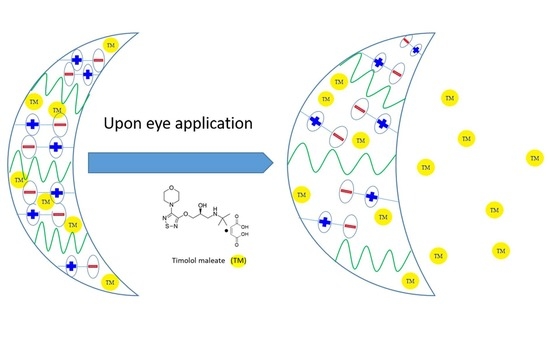
:1. Introduction
2. Results and Discussion
2.1. Equilibrium Swelling Ratio (ESR) of pSB-co-pVP Hydrogels
2.2. Elastic Modulus of pSB-co-pVP Hydrogels
2.3. Swelling Kinetics of pSB-co-pVP Networks
2.4. Differential Scanning Calorimetry (DSC) of pSB-co-pVP Networks
2.5. TM Entrapment Efficiency (EE) and Drug Loading Capacity (DLC) of pSB-co-pVP Hydrogels
2.6. TM Release from pSB-co-pVP Hydrogels
2.7. Kinetic Study on TM Release
2.8. Attenuated Total Teflectance Infrared Spectroscopy (ATR-IR)
2.9. Light Transmittance of TM-Loaded pSB-co-pVP Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of pSB-co-pVP Hydrogels and Neat pSB
4.2.2. Equilibrium Swelling Ratio (ESR)
4.2.3. Elastic Modulus of pSB-co-pVP Hydrogels
4.2.4. Swelling Kinetics
4.2.5. Timolol Maleate (TM) Loading in pSB-co-pVP Networks
4.2.6. Attenuated Total Reflectance Infrared Spectroscopy (ATR-IR)
4.2.7. Differential Scanning Calorimetry (DSC)
4.2.8. Light Transmittance
4.2.9. TM Entrapment Efficiency (EE) and Drug Loading Capacity (DLC)
4.2.10. TM Release Profiles
4.2.11. Kinetic Study of the TM in vitro Dissolution
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma therapy by extended release of Timolol from Nanoparticle Loaded Silicone-hydrogel contact lenses. J. Control. Release 2013, 165, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-C.; Chauhan, A. Modeling ophthalmic drug delivery by soaked contact lenses. Ind. Eng. Chem. Res. 2006, 45, 3718–3734. [Google Scholar] [CrossRef]
- Li, C.C.; Chauhan, A. Ocular Transport Model for ophthalmic delivery of timolol through P-Hema Contact lenses. J. Drug Deliv. Sci. Technol. 2007, 17, 69–79. [Google Scholar] [CrossRef]
- Hewitt, M.G.; Morrison, P.W.; Boostrom, H.M.; Morgan, S.R.; Fallon, M.; Lewis, P.N.; Whitaker, D.; Brancale, A.; Varricchio, C.; Quantock, A.J.; et al. In vitro topical delivery of chlorhexidine to the cornea: Enhancement using drug-loaded contact lenses and β-cyclodextrin complexation, and the importance of simulating tear irrigation. Mol. Pharm. 2020, 17, 1428–1441. [Google Scholar] [CrossRef]
- Minami, T.; Ishida, W.; Kishimoto, T.; Nakajima, I.; Hino, S.; Arai, R.; Matsunaga, T.; Fukushima, A.; Yamagami, S. In vitro and in vivo performance of epinastine hydrochloride-releasing contact lenses. PLoS ONE 2019, 14, e0210362. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.; Breaux, J.; Schentag, J.; Morck, D. Drug delivery to the posterior segment of the eye through Hydrogel Contact lenses. Clin. Exp. Optom. 2011, 94, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Patel, P.; Patel, G. Container closure selection and stability studies of developed multistimuli-responsive ocular sustained in situ hydrogel formulation of Timolol Maleate. J. Sol-Gel Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Pasi, S.; Archana, P.; Bhavana, E.; Maheshwari, R.; Mounika, E.; Manisha, A. Formulation and evaluation of mucoadhesive buccal patches of Timolol maleate. Int. J. Adv. Res. Med. Pharm. Sci. 2022, 6, e06439. [Google Scholar]
- Mirzaeei, S.; Faryadras, F.B.; Mehrandish, S.; Rezaei, L.; Daneshgar, F.; Karami, A. Development and evaluation of polycaprolactone-based electrospun nanofibers containing timolol maleate as a sustained-release device for treatment of glaucoma: In vivo evaluation in Equine Eye. Res. Pharm. Sci. 2022, 17, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, D.; Ruseva, K.; Tzachev, C.; Christov, L.; Vassileva, E. Novel Poly(Sulfobetaine methacrylate) based carriers as potential ocular drug delivery systems for timolol maleate. Polym. Int. 2022, 71, 662–667. [Google Scholar] [CrossRef]
- Ma, J.; Kang, K.; Yi, Q.; Zhang, Z.; Gu, Z. Multiple ph responsive zwitterionic micelles for stealth delivery of Anticancer Drugs. RSC Adv. 2016, 6, 64778–64790. [Google Scholar] [CrossRef]
- Lewoczko, E.M.; Wang, N.; Lundberg, C.E.; Kelly, M.T.; Kent, E.W.; Wu, T.; Chen, M.-L.; Wang, J.-H.; Zhao, B. Effects of n-substituents on the solution behavior of poly(sulfobetaine methacrylate)s in water: Upper and lower critical solution temperature transitions. ACS Appl. Polym. Mater. 2020, 3, 867–878. [Google Scholar] [CrossRef]
- Tkáčová, M.; Foffová, P. A Reference for Human Eye Surface Temperature Measurements in Diagnostic Process of Ophthalmologic Diseases: Semantic Scholar. Available online: https://www.semanticscholar.org/paper/A-Reference-for-Human-Eye-Surface-Temperature-in-of-Tk%C3%A1%C4%8Dov%C3%A1-Foffov%C3%A1/3f6dfa931f1a28638515a10022c99d0fcb3e4d1d (accessed on 15 December 2011).
- Rykowska, I.; Nowak, I.; Nowak, R. Soft contact lenses as drug delivery systems: A Review. Molecules 2021, 26, 5577. [Google Scholar] [CrossRef] [PubMed]
- Dunn, F.G.; Frohlich, E.D. Pharmacokinetics, mechanisms of action, indications, and adverse effects of timolol maleate, a nonselective beta-adrenoreceptor blocking agent. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1981, 1, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Taka, E.; Karavasili, C.; Bouropoulos, N.; Moschakis, T.; Andreadis, D.D.; Zacharis, C.K.; Fatouros, D.G. Ocular co-delivery of Timolol and brimonidine from a self-assembling peptide hydrogel for the treatment of glaucoma: In vitro and ex vivo evaluation. Pharmaceuticals 2020, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Rahma, A.; Munir, M.M.; Khairurrijal, K.; Prasetyo, A.; Suendo, V.; Rachmawati, H. Intermolecular interactions and the release pattern of Electrospun Curcumin-Polyvinyl(pyrrolidone) fiber. Biol. Pharm. Bull. 2016, 39, 163–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, S.; Ma, Y.; Chen, Y.; Li, D.; Cao, J.; Liu, Y.; Cai, M.; Xie, X.; Chen, Y.; Luo, X. Synthesis of an amphiphilic block copolymer containing Zwitterionic Sulfobetaine as a novel ph-sensitive drug carrier. Polym. Chem. 2014, 5, 1285–1297. [Google Scholar] [CrossRef]
- Saroj, A.L.; Singh, R.K.; Chandra, S. Studies on polymer electrolyte poly(vinyl) pyrrolidone (PVP) complexed with Ionic liquid: Effect of complexation on thermal stability, conductivity and relaxation behaviour. Mater. Sci. Eng. B 2013, 178, 231–238. [Google Scholar] [CrossRef]
- Acharya, A.; Goudanavar, P.; Joshi, V. Development and characterization of prolonged release timolol maleate cubosomal gel for Ocular Drug Delivery. Adv. Pharm. J. 2019, 4, 1–14. [Google Scholar] [CrossRef]
- Gulsen, D.; Chauhan, A. Dispersion of microemulsion drops in Hema Hydrogel: A Potential Ophthalmic Drug Delivery Vehicle. Int. J. Pharm. 2005, 292, 95–117. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, C.; Sun, Z.; Zhang, X.; Liang, N.; Mao, S. Inner layer-embedded contact lenses for PH-triggered controlled ocular drug delivery. Eur. J. Pharm. Biopharm. 2018, 128, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Giblin, F.J.; Lin, L.-R.; Leverenz, V.R.; Dang, L. A Class I (Senofilcon A) Soft Contact Lens Prevents UVB-Induced Ocular Effects, Including Cataract, in the Rabbit In Vivo. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3667–3675. [Google Scholar] [CrossRef]
- Schwarz, U.D. A generalized analytical model for the elastic deformation of an adhesive contact between a sphere and a flat surface. J. Colloid Interface Sci. 2003, 261, 99–106. [Google Scholar] [CrossRef]
- Wagner, M. Determination of Water Content and Dynamic Vapor Sorption Using. Available online: https://www.researchgate.net/publication/305154370_Determination_of_Water_Content_and_Dynamic_Vapor_Sorption_Using_Gravimetric_Methods_Karl_Fischer_Titration_and_Thermal_Analysis (accessed on 20 June 2016).
- Baishya, H. Application of mathematical models in drug release kinetics of carbidopa and levodopa er tablets. J. Dev. Drugs 2017, 6, 171. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–1047. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release i. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]








| Sample Designation | ESR |
|---|---|
| SV1-2 | 2.55 ± 0.02 |
| SV1-1 | 2.2 ± 0.03 |
| SV2-1 | 1.98 ± 0.02 |
| pSB | 1.72 ± 0.04 |
| Sample Designation | Elastic Modulus [MPa] |
|---|---|
| SV1-2 | 8.82 ± 0.04 |
| SV1-1 | 8.78 ± 0.09 |
| SV2-1 | 9.07 ± 0.05 |
| pSB | 9.22 ± 0.04 |
| Sample | Wc of Non-Loaded Samples [%] | Wc of TM-Loaded Samples [%] |
|---|---|---|
| SV1-2 | 29.5% | 38.6% |
| SV1-1 | 27.8% | 42.3% |
| SV2-1 | 25.0% | 24.5% |
| pSB | 27.1% | 20.2% |
| Model/Sample | SV1-2 | SV1-1 | SV2-1 | pSB | |
|---|---|---|---|---|---|
| Zero order | K0 | 1.023 | 1.826 | 1.163 | 1.227 |
| R02 | 0.775 | 0.739 | 0.619 | 0.644 | |
| First order | K1 | 0.005 | 0.011 | 0.0069 | 0.008 |
| R12 | 0.408 | 0.662 | 0.584 | 0.574 | |
| Higuchi model | KH | 6.721 | 9.268 | 8.974 | 7.321 |
| RH2 | 0.678 | 0.691 | 0.812 | 0.829 | |
| Korsmeyer-Peppas | KKP | - | 54.475 | 70.958 | 56.079 |
| n | - | 0.104 | 0.162 | 0.099 | |
| RKP2 | - | 0.551 | 0.998 | 0.913 | |
| Sample | UV-B (280–315 nm) | UV-A (316–380 nm) | SWB * (381–460 nm) | LWB ** (461–500 nm) |
|---|---|---|---|---|
| SV1-2 | 28.55% | 81.43% | 85.61% | 85.84% |
| SV1-1 | 27.53% | 72.12% | 74.78% | 75.06% |
| SV2-1 | 22.48% | 82.58% | 85.16% | 85.5% |
| pSB | 19.16% | 77.12% | 79.22% | 79.70% |
| Sample Designation | SB (Molar Parts) | VP (Molar Parts) |
|---|---|---|
| SV1-2 | 1 | 2 |
| SV1-1 | 1 | 1 |
| SV2-1 | 2 | 1 |
| pSB | 1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolova, D.; Tzachev, C.; Christov, L.; Vassileva, E. Poly(Sulfobetaine Methacrylate-co-Vinyl Pyrrolidone) Hydrogels as Potential Contact Lenses Delivery Systems for Timolol Maleate. Gels 2023, 9, 114. https://doi.org/10.3390/gels9020114
Nikolova D, Tzachev C, Christov L, Vassileva E. Poly(Sulfobetaine Methacrylate-co-Vinyl Pyrrolidone) Hydrogels as Potential Contact Lenses Delivery Systems for Timolol Maleate. Gels. 2023; 9(2):114. https://doi.org/10.3390/gels9020114
Chicago/Turabian StyleNikolova, Denitsa, Christo Tzachev, Lachezar Christov, and Elena Vassileva. 2023. "Poly(Sulfobetaine Methacrylate-co-Vinyl Pyrrolidone) Hydrogels as Potential Contact Lenses Delivery Systems for Timolol Maleate" Gels 9, no. 2: 114. https://doi.org/10.3390/gels9020114
APA StyleNikolova, D., Tzachev, C., Christov, L., & Vassileva, E. (2023). Poly(Sulfobetaine Methacrylate-co-Vinyl Pyrrolidone) Hydrogels as Potential Contact Lenses Delivery Systems for Timolol Maleate. Gels, 9(2), 114. https://doi.org/10.3390/gels9020114