Bioinspired Oxidation-Resistant Catechol-like Sliding Ring Polyrotaxane Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
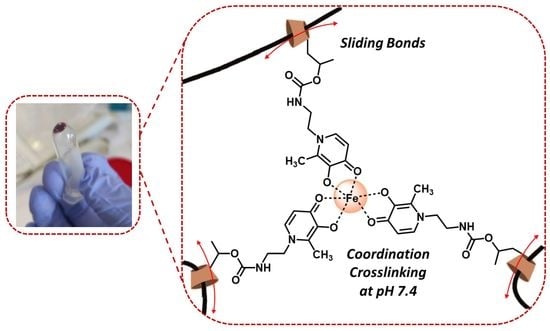
2.1. Preparation and Characterization of Novel Polyrotaxane-Hydroxypyridinones (PR-HOPO) Network
2.2. Preparation and Characterization of PR-HOPO Hydrogels
2.3. Assessment of Cell Viability upon Contact with PR-HOPO Formulations
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of Novel Polyrotaxane-Hydroxypyridinones (PR-HOPO)
4.2.1. General Procedure for the Preparation of HOPO 4a and 4b
4.2.2. General Procedure for Polyrotaxane-Hydroxypyridinones (PR-HOPO) Functionalization
4.2.3. Determination of the Degree of Substitution (DS)
4.3. Preparation and Characterization of HOPO-PR Hydrogels
4.4. Assessment of Cell Viability upon Contact with PR-HOPO Formulations
4.5. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, A.; Chen, Y.; Birkedal, H. Bioinspired metal-polyphenol materials: Self-healing and beyond. Biomimetics 2019, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, S.; Costa, A.M.S.; Andersen, A.; Choi, I.S.; Birkedal, H.; Mano, J.F. Bioinspired Ultratough Hydrogel with Fast Recovery, Self-Healing, Injectability and Cytocompatibility. Adv. Mater. 2017, 29, 1700759. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.; Krogsgaard, M.; Birkedal, H. Mussel-Inspired Self-Healing Double-Cross-Linked Hydrogels by Controlled Combination of Metal Coordination and Covalent Cross-Linking. Biomacromolecules 2018, 19, 1402–1409. [Google Scholar] [CrossRef] [Green Version]
- Krogsgaard, M.; Nue, V.; Birkedal, H. Mussel-Inspired Materials: Self-Healing through Coordination Chemistry. Chem. Eur. J. 2016, 22, 844–857. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mian, S.A.; Gao, X.; Nagase, S.; Jang, J. Adsorption of catechol on a wet silica surface: Density functional theory study. Theor. Chem. Acc. 2011, 130, 333–339. [Google Scholar] [CrossRef]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Yavvari, P.S.; Srivastava, A. Robust, self-healing hydrogels synthesised from catechol rich polymers. J. Mater. Chem. B 2015, 3, 899–910. [Google Scholar] [CrossRef]
- Mamidi, N.; García, R.G.; Martínez, J.D.H.; Briones, C.M.; Martínez Ramos, A.M.; Tamez, M.F.L.; Del Valle, B.G.; Segura, F.J.M. Recent Advances in Designing Fibrous Biomaterials for the Domain of Biomedical, Clinical, and Environmental Applications. ACS Biomater. Sci. Eng. 2022, 8, 3690–3716. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V. Design, fabrication and drug release potential of dual stimuli-responsive composite hydrogel nanoparticle interfaces. Colloids Surf. B Biointerfaces 2021, 204, 111819. [Google Scholar] [CrossRef]
- Rial-Hermida, M.I.; Rey-Rico, A.; Blanco-Fernandez, B.; Carballo-Pedrares, N.; Byrne, E.M.; Mano, J.F. Recent Progress on Polysaccharide-Based Hydrogels for Controlled Delivery of Therapeutic Biomolecules. ACS Biomater. Sci. Eng. 2021, 7, 4102–4127. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Heilshorn, S.C. Adaptable Hydrogel Networks with Reversible Linkages for Tissue Engineering. Adv. Mater. 2015, 27, 3717–3736. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, Y. Weak bond-based injectable and stimuli responsive hydrogels for biomedical applications. J. Mater. Chem. B 2017, 5, 887–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyot, C.; Cerruti, M.; Lerouge, S. Injectable, strong and bioadhesive catechol-chitosan hydrogels physically crosslinked using sodium bicarbonate. Mater. Sci. Eng. C 2021, 118, 111529. [Google Scholar] [CrossRef] [PubMed]
- Holten-Andersen, N.; Jaishankar, A.; Harrington, M.J.; Fullenkamp, D.E.; Dimarco, G.; He, L.; McKinley, G.H.; Messersmith, P.B.; Lee, K.Y.C. Metal-coordination: Using one of nature’s tricks to control soft material mechanics. J. Mater. Chem. B 2014, 2, 2467–2472. [Google Scholar] [CrossRef] [Green Version]
- Agergaard, A.H.; Pedersen, S.U.; Birkedal, H.; Daasbjerg, K. Stimuli-responsive degrafting of polymer brushes: Via addressable catecholato-metal attachments. Polym. Chem. 2020, 11, 5572–5577. [Google Scholar] [CrossRef]
- Shafiq, Z.; Cui, J.; Pastor-Pérez, L.; San Miguel, V.; Gropeanu, R.A.; Serrano, C.; del Campo, A. Bioinspired underwater bonding and debonding on demand. Angew. Chem.-Int. Ed. 2012, 51, 4332–4335. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ejima, H.; Yoshie, N. Seawater-Assisted Self-Healing of Catechol Polymers via Hydrogen Bonding and Coordination Interactions. ACS Appl. Mater. Interfaces 2016, 8, 19047–19053. [Google Scholar] [CrossRef]
- Costa, A.M.S.; Mano, J.F. Highly robust hydrogels via a fast, simple and cytocompatible dual crosslinking-based process. Chem. Commun. 2015, 51, 15673–15676. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Liu, C.; Jiang, L.; Lin, J.; Qian, J.; Mayumi, K.; Wu, Z.L.; Ito, K.; Zheng, Q. Slide-Ring Cross-Links Mediated Tough Metallosupramolecular Hydrogels with Superior Self-Recoverability. Macromolecules 2019, 52, 6748–6755. [Google Scholar] [CrossRef]
- Neto, A.I.; Vasconcelos, N.L.; Oliveira, S.M.; Ruiz-Molina, D.; Mano, J.F. High-Throughput Topographic, Mechanical, and Biological Screening of Multilayer Films Containing Mussel-Inspired Biopolymers. Adv. Funct. Mater. 2016, 26, 2745–2755. [Google Scholar] [CrossRef] [Green Version]
- Nakahata, M.; Mori, S.; Takashima, Y.; Yamaguchi, H.; Harada, A. Self-Healing Materials Formed by Cross-Linked Polyrotaxanes with Reversible Bonds. Chem 2016, 1, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Hart, L.F.; Tranquilli, M.M.; Rowan, S.J. Material properties and applications of mechanically interlocked polymers. Nat. Rev. Mater. 2021, 6, 508–530. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Kobayashi, Y. Precise synthesis of polyrotaxane and preparation of supramolecular materials based on its mobility. Polym. J. 2021, 53, 505–513. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Zheng, Y.; Takashima, Y.; Yamaguchi, H.; Harada, A. Physical and adhesion properties of supramolecular hydrogels cross-linked by movable cross-linking molecule and host-guest interactions. Chem. Lett. 2018, 47, 1387–1390. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, N.; Seo, J.H.; Tamura, A.; Sasaki, Y.; Yui, N. Tailoring the supramolecular structure of aminated polyrotaxanes toward enhanced cellular internalization. Macromol. Biosci. 2014, 14, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Menyo, M.S.; Hawker, C.J.; Waite, J.H. Versatile tuning of supramolecular hydrogels through metal complexation of oxidation-resistant catechol-inspired ligands. Soft Matter 2013, 9, 10314–10323. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.C.; Costa, D.C.S.; Oliveira, C.S.; Mano, J.F. Design of Protein-Based Liquefied Cell-Laden Capsules with Bioinspired Adhesion for Tissue Engineering. Adv. Healthc. Mater. 2021, 10, 2100782. [Google Scholar] [CrossRef]
- Mawani, Y.; Cawthray, J.F.; Chang, S.; Sachs-Barrable, K.; Weekes, D.M.; Wasan, K.M.; Orvig, C. In vitro studies of lanthanide complexes for the treatment of osteoporosis. Dalt. Trans. 2013, 42, 5999–6011. [Google Scholar] [CrossRef]
- Mayumi, K.; Liu, C.; Yasuda, Y.; Ito, K. Softness, elasticity, and toughness of polymer networks with slide-ring cross-links. Gels 2021, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Mayumi, K.; Ito, K. Structure and dynamics of polyrotaxane and slide-ring materials. Polymer 2010, 51, 959–967. [Google Scholar] [CrossRef] [Green Version]
- Maier, G.P.; Bernt, C.M.; Butler, A. Catechol oxidation: Considerations in the design of wet adhesive materials. Biomater. Sci. 2018, 6, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.J.R.; Gaspar, V.M.; Mano, J.F. Responsive laminarin-boronic acid self-healing hydrogels for biomedical applications. Polym. J. 2020, 52, 997–1006. [Google Scholar] [CrossRef]
- Minato, K.; Mayumi, K.; Maeda, R.; Kato, K.; Yokoyama, H.; Ito, K. Mechanical properties of supramolecular elastomers prepared from polymer-grafted polyrotaxane. Polymer 2017, 128, 386–391. [Google Scholar] [CrossRef]
- Barrett, D.G.; Fullenkamp, D.E.; He, L.; Holten-andersen, N.; Lee, K.Y.C.; Messersmith, P.B. pH-Based Regulation of Hydrogel Mechanical Properties through Mussel-Inspired Chemistry and Processing. Adv. Funct. Mater. 2013, 23, 1111–1119. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rial-Hermida, M.I.; Costa, D.C.S.; Jiang, L.; Rodrigues, J.M.M.; Ito, K.; Mano, J.F. Bioinspired Oxidation-Resistant Catechol-like Sliding Ring Polyrotaxane Hydrogels. Gels 2023, 9, 85. https://doi.org/10.3390/gels9020085
Rial-Hermida MI, Costa DCS, Jiang L, Rodrigues JMM, Ito K, Mano JF. Bioinspired Oxidation-Resistant Catechol-like Sliding Ring Polyrotaxane Hydrogels. Gels. 2023; 9(2):85. https://doi.org/10.3390/gels9020085
Chicago/Turabian StyleRial-Hermida, M. Isabel, Dora C. S. Costa, Lan Jiang, João M. M. Rodrigues, Kohzo Ito, and João F. Mano. 2023. "Bioinspired Oxidation-Resistant Catechol-like Sliding Ring Polyrotaxane Hydrogels" Gels 9, no. 2: 85. https://doi.org/10.3390/gels9020085
APA StyleRial-Hermida, M. I., Costa, D. C. S., Jiang, L., Rodrigues, J. M. M., Ito, K., & Mano, J. F. (2023). Bioinspired Oxidation-Resistant Catechol-like Sliding Ring Polyrotaxane Hydrogels. Gels, 9(2), 85. https://doi.org/10.3390/gels9020085