Effect of Hogweed Pectin on Rheological, Mechanical, and Sensory Properties of Apple Pectin Hydrogel
Abstract
:1. Introduction
2. Results and Discussion
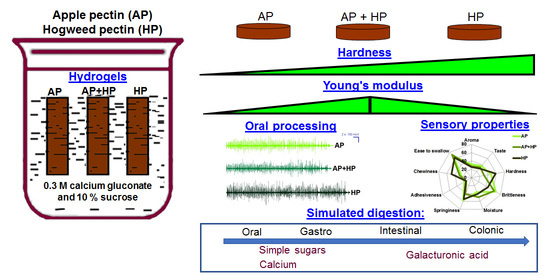
2.1. Preparation of Pectin Hydrogels
2.2. Rheological Properties of Pectin Hydrogels
2.3. Mechanical Properties of Pectin Hydrogels
2.4. Oral Processing and Sensory Evaluation of Pectin Hydrogels
2.5. Correlation Analysis of Mechanical, Rheological, and Sensory Properties of Pectin Hydrogels
2.6. Simulated Digestibility of Pectin Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Pectin Hydrogels
4.3. Characterization of Pectin Hydrogels
4.3.1. General Characterization
4.3.2. Rheological Characterization of Pectin Hydrogels
4.3.3. Instrumental Texture Characterization of Pectin Hydrogels
4.3.4. Characterization of Oral Processing of Pectin Hydrogels
4.3.5. In Vivo Oral Phase (OP) and Static In Vitro Gastrointestinal Digestion
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nath, P.C.; Debnath, S.; Sridhar, K.; Inbaraj, B.S.; Nayak, P.K.; Sharma, M. A Comprehensive Review of Food Hydrogels: Principles, Formation Mechanisms, Microstructure, and Its Applications. Gels 2023, 9, 1. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Bandral, J.D.; Gani, A.; Shams, R. Food hydrocolloids: Functional, nutraceutical and novel applications for delivery of bioactive compounds. Int. J. Biol. Macromol. 2020, 165, 554–567. [Google Scholar] [CrossRef]
- Yang, Y.; Anderson, C.T. Biosynthesis, localisation, and function of pectins in plants. In Pectin: Technological and Physiological Properties; Springer: Cham, Switzerland, 2020; pp. 1–15. [Google Scholar]
- Padma Ishwarya, S.; Sandhya, R.; Nisha, P. Advances and prospects in the food applications of pectin hydrogels. Crit. Rev. Food Sci. Nutr. 2021, 62, 4393–4417. [Google Scholar] [CrossRef] [PubMed]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin—A review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological activity and pharmacological application of pectic polysaccharides: A review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [Green Version]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odun-Ayo, F.; Reddy, L. Potential Biomedical Applications of Modified Pectin as a delivery system for bioactive substances. Polysaccharides 2023, 4, 1–32. [Google Scholar] [CrossRef]
- Belkheiri, A.; Forouhar, A.; Ursu, A.V.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.; Abdelkafi, S.; Hamdami, N.; Michaud, P. Extraction, Characterization, and Applications of Pectins from Plant By-Products. Appl. Sci. 2021, 11, 6596. [Google Scholar] [CrossRef]
- Ropartz, D.; Ralet, M.-C. Pectin structure. In Pectin: Technological and Physiological Properties; Kontogiorgos, V., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 17–36. [Google Scholar]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, B.; Nakauma, M.; Funami, T.; Nishinari, K.; Draget, K.I.; Phillips, G.O.; Fang, Y. Modulation of calcium-induced gelation of pectin by oligoguluronate as compared to alginate. Food Res. Int. 2018, 116, 232–240. [Google Scholar] [CrossRef]
- Makarova, E.N.; Shakhmatov, E.G.; Belyy, V.A. Structural characteristics of oxalate-soluble polysaccharides of Sosnowsky’s hogweed (Heracleum sosnowskyi Manden). Carbohydr. Polym. 2016, 153, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Patova, O.A.; Golovchenko, V.V.; Vityazev, F.V.; Burkov, A.A.; Belyi, V.A.; Kuznetsov, S.N.; Litvinets, S.G.; Martinson, E.A. Physicochemical and rheological properties of gelling pectin from Sosnowskyi’s hogweed (Heracleum sosnowskyi) obtained using different pretreatment conditions. Food Hydrocoll. 2017, 65, 77–86. [Google Scholar] [CrossRef]
- Popov, S.; Paderin, N.; Khramova, D.; Kvashninova, E.; Patova, O.; Vityazev, F. Swelling, Protein Adsorption, and Biocompatibility In Vitro of Gel Beads Prepared from Pectin of Hogweed Heracleum sosnówskyi Manden in Comparison with Gel Beads from Apple Pectin. Int. J. Mol. Sci. 2022, 23, 3388. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef] [PubMed]
- Mala, T.; Anal, A.K. Protection and Controlled Gastrointestinal Release of Bromelain by Encapsulating in Pectin–Resistant Starch Based Hydrogel Beads. Front. Bioeng. Biotechnol. 2021, 9, 757176. [Google Scholar] [CrossRef]
- Tarifa, M.C.; Piqueras, C.M.; Genovese, D.B.; Brugnoni, L.I. Microencapsulation of Lactobacillus casei and Lactobacillus rhamnosus in pectin and pectin-inulin microgel particles: Effect on bacterial survival under storage conditions. Int. J. Biol. Macromol. 2021, 179, 457–465. [Google Scholar] [CrossRef]
- Lee, T.; Chang, Y.H. Structural, physicochemical, and in-vitro release properties of hydrogel beads produced by oligochitosan and de-esterified pectin from yuzu (Citrus junos) peel as a quercetin delivery system for colon target. Food Hydrocoll. 2020, 108, 106086. [Google Scholar] [CrossRef]
- Alghooneh, A.; Razavi, S.M.A.; Kasapis, S. Classification of hydrocolloids based on small amplitude oscillatory shear, large amplitude oscillatory shear, and textural properties. J. Texture Stud. 2019, 50, 520–538. [Google Scholar] [CrossRef]
- Funami, T.; Nakauma, M. Instrumental food texture evaluation in relation to human perception. Food Hydrocoll. 2022, 124, 107253. [Google Scholar] [CrossRef]
- Brighenti, M.; Govindasamy-Lucey, S.; Jaeggi, J.J.; Johnson, M.E.; Lucey, J.A. Effects of processing conditions on the texture and rheological properties of model acid gels and cream cheese. J. Dairy Sci. 2018, 101, 6762–6775. [Google Scholar] [CrossRef]
- Koc, H.; Vinyard, C.J.; Essick, G.K.; Foegeding, E.A. Food oral processing: Conversion of food structure to textural perception. Annu. Rev. Food Sci. Technol. 2013, 4, 237–266. [Google Scholar] [CrossRef] [PubMed]
- De Lavergne, M.D.; Van de Velde, F.; Stieger, M. Bolus matters: The influence of food oral breakdown on dynamic texture perception. Food Funct. 2017, 8, 464–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid, M.; Yaich, H.; Hidouri, H.; Attia, H.; Ayadi, M.A. Effect of substituted gelling agents from pomegranate peel on colour, textural and sensory properties of pomegranate jam. Food Chem. 2018, 239, 1047–1054. [Google Scholar] [CrossRef]
- Basu, S.; Shivhare, U.S. Rheological, textural, micro-structural and sensory properties of mango jam. J. Food Eng. 2010, 100, 357–365. [Google Scholar] [CrossRef]
- Puleo, S.; Valentino, M.; Masi, P.; Di Monaco, R. Hardness sensitivity: Are old, young, female and male subjects all equally sensitive? Food Qual. Prefer. 2021, 90, 104118. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nakauma, M.; Funami, T.; Hori, K.; Ono, T. Human physiological responses during swallowing of gel-type foods and its correlation with textural perception. Food Hydrocoll. 2021, 111, 106353. [Google Scholar] [CrossRef]
- Sorapukdee, S.; Jansa, S.; Tangwatcharin, P. Partial replacement of pork backfat with konjac gel in Northeastern Thai fermented sausage (Sai Krok E-san) to produce the healthier product. Asian-Australas J. Anim. Sci. 2019, 32, 1763–1775. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Feng, Y.; Kong, B.; Xia, X.; Liu, M.; Chen, J.; Zhang, F.; Liu, Q. Textural and gel properties of frankfurters as influenced by various κ-carrageenan incorporation methods. Meat Sci. 2021, 176, 108483. [Google Scholar] [CrossRef]
- Petcharat, T.; Benjakul, S. Effect of gellan incorporation on gel properties of bigeye snapper surimi. Food Hydrocoll. 2018, 77, 746–753. [Google Scholar] [CrossRef]
- Tan, H.-L.; Tan, T.C.; Easa, A.M. The use of selected hydrocolloids and salt substitutes on structural integrity, texture, sensory properties, and shelf life of fresh no salt wheat noodles. Food Hydrocoll. 2020, 108, 105996. [Google Scholar] [CrossRef]
- Nepovinnykh, N.V.; Kliukina, O.N.; Ptichkina, N.; Bostan, A. Hydrogel based dessert of low calorie content. Food Hydrocoll. 2019, 86, 184–192. [Google Scholar] [CrossRef]
- Ford, C.G.; Bolhuis, D. Interrelations Between Food Form, Texture, and Matrix Influence Energy Intake and Metabolic Responses. Curr. Nutr. Rep. 2022, 11, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Ngouémazong, D.E.; Kabuye, G.; Fraeye, I.; Cardinaels, R.; Van Loey, A.; Moldenaers, P.; Hendrickx, M. Effect of debranching on the rheological properties of Ca2+ pectin gels. Food Hydrocoll. 2012, 26, 44–53. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J.; Zhang, H.; Wu, D.; Ye, X.; Linardt, R.J.; Chen, S. Gelling mechanism of RG-I enriched citrus pectin: Role of arabinose side-chains in cation- and acid-induced gelation. Food Hydrocoll. 2020, 101, 105536. [Google Scholar] [CrossRef]
- Khramova, D.S.; Popov, S.V. A secret of salivary secretions: Multimodal effect of saliva in sensory perception of food. Eur. J. Oral Sci. 2022, 130, e12846. [Google Scholar] [CrossRef]
- Devezeaux de Lavergne, M.; Strijbosch, V.M.G.; Van den Broek, A.W.M.; Van de Velde, F.; Stieger, M. Uncoupling the Impact of fracture properties and composition on sensory perception of emulsion-filled gels. J. Texture Stud. 2016, 47, 92–111. [Google Scholar] [CrossRef]
- Devezeaux de Lavergne, M.; Van de Velde, F.; Van Boekel, M.A.J.S.; Stieger, M. Dynamic texture perception and oral processing of semi-solid food gels: Part 2: Impact of breakdown behaviour on bolus properties and dynamic texture perception. Food Hydrocoll. 2015, 49, 61–72. [Google Scholar] [CrossRef]
- Bongaerts, J.H.H.; Rossetti, D.; Stokes, J.R. The Lubricating Properties of Human Whole Saliva. Tribol Lett. 2007, 27, 277–287. [Google Scholar] [CrossRef]
- Funami, T.; Nakao, S.; Isono, M.; Ishihara, S.; Nakauma, M. Effects of food consistency on perceived intensity and eating behavior using soft gels with varying aroma inhomogeneity. Food Hydrocoll. 2016, 52, 896–905. [Google Scholar] [CrossRef]
- Krop, E.M.; Hetherington, M.M.; Holmes, M.; Miquel, S.; Sarkar, A. On relating rheology and oral tribology to sensory properties in hydrogels. Food Hydrocoll. 2019, 88, 101–113. [Google Scholar] [CrossRef]
- Methacanon, P.; Gamonpilas, C.; Kongjaroen, A.; Buathongjan, C. Food polysaccharides and roles of rheology and tribology in rational design of thickened liquids for oropharyngeal dysphagia: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4101–4119. [Google Scholar] [CrossRef] [PubMed]
- Le Bleis, F.; Chaunier, L.; Della Valle, G.; Panouillé, M.; Réguerre, A.-L. Physical assessment of bread destructuration during chewing. Food Res. Int. 2012, 50, 308–317. [Google Scholar] [CrossRef]
- Panouillé, M.; Saint-Eve, A.; Déléris, I.; Le Bleis, F.; Souchon, I. Oral processing and bolus properties drive the dynamics of salty and texture perceptions of bread. Food Res. Int. 2014, 62, 238–246. [Google Scholar] [CrossRef]
- Kurotobi, T.; Hoshino, T.; Kazami, Y.; Hayakawa, F.; Hagura, Y. Relationship between sensory analysis for texture and instrument measurements in model strawberry jam. J. Texture Stud. 2018, 49, 359–369. [Google Scholar] [CrossRef]
- Stribitcaia, E.; Krop, E.M.; Lewin, R.; Holmes, M.; Sarkar, A. Tribology and rheology of bead-layered hydrogels: Influence of bead size on sensory perception. Food Hydrocoll. 2020, 104, 105692. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, K.; Descallar, F.B.A.; Li, A.; Yang, X.; Yang, H. Gelation behaviors of some special plant-sourced pectins: A review inspired by examples from traditional gel foods in China. Trends Food Sci. Technol. 2022, 126, 26–40. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Shangguan, X.; Wang, H.; Sha, X.; Bansal, N. Rheological behavior, emulsifying properties and structural characterization of phosphorylated fish gelatin. Food Chem. 2018, 246, 428–436. [Google Scholar] [CrossRef]
- Holdsworth, S.D. Applicability of rheological models to the interpretation of flow and processing behavior of fluid food products. J. Texture Stud. 1971, 2, 393–418. [Google Scholar] [CrossRef]
- Ramkumar, D.H.S.; Bhattacharya, M. Relaxation behavior and the application of integral constitutive equations to wheat dough. J. Texture Stud. 1996, 27, 517–544. [Google Scholar] [CrossRef]
- Gabriele, D.G.; Migliori, M.; Sanzo, R.D.; Rossi, C.O.; Ruffolo, S.A.; Cindio, B. Characterization of dairy emulsions by NMR and rheological techniques. Food Hydrocoll. 2009, 23, 619–628. [Google Scholar] [CrossRef]
- Popov, S.; Smirnov, V.; Paderin, N.; Khramova, D.; Chistiakova, E.; Vityazev, F.; Golovchenko, V. Enrichment of Agar Gel with Antioxidant Pectin from Fireweed: Mechanical and Rheological Properties, Simulated Digestibility, and Oral Processing. Gels 2022, 8, 708. [Google Scholar] [CrossRef] [PubMed]
- Lawless, H.; Heymann, H. Introduction. In Sensory Evaluation of Food; Food Science Text Series; Springer: New York, NY, USA, 2010. [Google Scholar]
- Choy, J.Y.M.; Goh, A.T.; Chatonidi, G.; Ponnalagu, S.; Wee, S.M.M.; Stieger, M.; Forde, C.G. Impact of food texture modifications on oral processing behaviour, bolus properties and postprandial glucose responses. Curr. Res. Food Sci. 2021, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Khin, M.N.; Goff, H.D.; Nsor-Atindana, J.; Ahammed, S.; Liu, F.; Zhong, F. Effect of texture and structure of polysaccharide hydrogels containing maltose on release and hydrolysis of maltose during digestion: In vitro study. Food Hydrocoll. 2021, 112, 106326. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I.; Klochkova, N.G. Polysaccharides of algae. 48. Polysaccharide composition of several Calcareous red algae: Isolation of alginate from Corallina Pilulifera P. et R. (Rhodophyta, Corallinaceae). Bot. Mar. 1995, 38, 43–51. [Google Scholar] [CrossRef]








| Parameters | PG1 | PG2 | PG3 | PG4 | PG5 |
|---|---|---|---|---|---|
| G′LVE (Pa) | 51,636 ± 4741 a | 61,209 ± 7204 b | 74,486 ± 10,993 c | 88,753 ± 5467 d | 98,533 ± 14,595 e |
| G″LVE (Pa) | 10,966 ± 2033 a | 12,997 ± 3039 a | 16,562 ± 4640 b | 17,428 ± 2411 c | 25,357 ± 4587 d |
| G*LVE | 53,643 ± 5536 a | 63,046 ± 5936 b | 77,100 ± 9036 c | 89,529 ± 6592 d | 101,313 ± 15,488 e |
| Tan [δ]LVE | 0.21 ± 0.04 a | 0.22 ± 0.09 a | 0.24 ± 0.12 a | 0.20 ± 0.04 a | 0.27 ± 0.10 a |
| γL (%) | 0.35 ± 0.08 a | 0.23 ± 0.02 a,b | 0.20 ± 0.01 a,b | 0.19 ± 0.02 b | 0.15 ± 0.04 b |
| τFr (Pa) | 251 ± 151 a | 155 ± 48 a | 170 ± 18 a | 216 ± 42 a | 114 ± 24 a |
| γFr | 0.75 ± 0.10 a | 0.54 ± 0.03 b | 0.48 ± 0.05 b,c | 0.44 ± 0.01 c | 0.30 ± 0.06 d |
| G*FP (Pa) | 24,252 ± 9158 a | 29,605 ± 8375 a | 38,404 ± 5752 a | 47,546 ± 9452 a | 53,702 ± 39,039 a |
| Tan [δ]AF | 0.34 ± 0.13 a,c,d | 0.36 ± 0.12 a,b,c,d | 0.66 ± 0.06 b | 0.39 ± 0.06 a,c | 0.19 ± 0.04 a,d |
| G*max/G*LVE | 1.25 ± 0.21 a | 1.26 ± 0.17 a | 1.20 ± 0.02 a | 1.05 ± 0.08 a | 1.53 ± 0.18 a |
| Parameters | PG1 | PG2 | PG3 | PG4 | PG5 | ||
|---|---|---|---|---|---|---|---|
| k′ (Pa*s) | 71,741 ± 20,277 a | 86,851 ± 9022 a | 88,590 ± 3574 a | 83,490 ± 32,656 a,b | 127,855 ± 15,415 b | ||
| k″ (Pa*s) | 10,307 ± 2719 a | 11,295 ± 2339 a | 13,227 ± 92 a,b | 15,140 ± 4187 a,b | 19,114 ± 5748 b | ||
| A | 75,313 ± 16,622 a | 83,298 ± 5382 a | 84,316 ± 4866 | 101,487 ± 37,040 a,b | 135,162 ± 25,722 b | ||
| k″/k′ | 0.14 ± 0.00 a | 0.13 ± 0.02 a | 0.15 ± 0.01 a,b | 0.19 ± 0.03 b | 0.17 ± 0.02 a,b | ||
| η*s | 12,039 ± 2919 a | 12,701 ± 2685 a | 10,096 ± 4678 a | 12,346 ± 4791 a | 18,773 ± 6072 a | ||
| n′ | 0.08 ± 0.02 a | 0.09 ± 0.02 a | 0.10 ± 0.02 a | 0.08 ± 0.05 a | 0.09 ± 0.01 a | ||
| n″ | 0.10 ± 0.02 a,c | 0.10 ± 0.01 a | 0.08 ± 0.01 a,c | 0.04 ± 0.01 b | 0.08 ± 0.05 a,b,c | ||
| z | 14.2 ± 3.7 a | 10.8 ± 3.0 a | 9.6 ± 2.4 a | 9.3 ± 1.2 a | 9.7 ± 1.3 a | ||
| Frequency (Hz) | 0.54 | G′ (kPa) | 72.8 ± 18.1 a | 74.4 ± 15.7 a,b | 62.3 ± 29.5 ab | 78.7 ± 32.3 a,b | 118.9 ± 25.7 b |
| G″ (kPa) | 11.3 ± 2.4 a | 12.9 ± 3.7 a,b | 12.9 ± 4.2 b | 19.6 ± 2.9 b | 31.8 ± 6.3 c | ||
| 1.11 | G′ (kPa) | 72.4 ± 20.7 a | 81.0 ± 17.0 a | 69.1 ± 28.0 a | 89.2 ± 32.7 a | 132.5 ± 27.6 b | |
| G″ (kPa) | 10.4 ± 2.9 a | 11.1 ± 2.1 a,b | 10.7 ± 4.0 ab | 13.7 ± 3.7 a,b | 19.2 ± 6.2 b | ||
| 10.50 | G′ (kPa) | 87.1 ± 26.7 a | 96.9 ± 19.4 a | 84.0 ± 34.8 a | 100.4 ± 34.8 a,b | 174.2 ± 36.6 b | |
| G″ (kPa) | 13.0 ± 4.1 a | 14.1 ± 2.9 a | 12.1 ± 4.8 a | 16.8 ± 5.1 a,b | 26.9 ± 5.6 b | ||
| 30.60 | G′ (kPa) | 94.7 ± 31.6 a | 108.3 ± 22.7 a | 93.0 ± 38.9 a | 100.9 ± 37.7 a | 192.0 ± 36.4 b | |
| G″ (kPa) | 15.7 ± 5.9 a | 16.0 ± 3.5 a | 13.7 ± 5.6 a | 17.8 ± 5.8 a,b | 30.7 ± 8.8 b | ||
| Hydrogel | Viscosity | ||||
|---|---|---|---|---|---|
| K (Pa*s) | R2 | n | ηapp 10 (Hz) | ηapp 50 (Hz) | |
| PG1 | 11,942 | 0.999 | −0.946 | 1333.10 ± 409.6 | 273.9 ± 109.4 |
| PG2 | 12,677 | 0.999 | −0.904 | 1482.5 ± 297.0 | 343.6 ± 79.6 |
| PG3 | 10,780 | 0.999 | −0.899 | 1285.1 ± 531.2 | 291.4 ± 125.1 |
| PG4 | 13,310 | 0.997 | −0.926 | 1541.4 ± 532.7 | 303.2 ± 111.2 |
| PG5 | 18,847 | 0.999 | −0.908 | 2162.8 ± 872.7 | 502.0 ± 198.3 |
| Hydrogel | Hardness (N) | Fracturability (N) | Consistency (mJ) | Adhesiveness (mN) | Brittleness (mm) | Young’s Modulus (kPa) |
|---|---|---|---|---|---|---|
| PG1 | 3.09 ± 0.41 a | 3.09 ± 0.41 a | 3.90 ± 0.51 a | 30.1 ± 3.4 a | 1.9 ± 0.2 a | 608 ± 52 a |
| PG2 | 2.63 ± 0.49 a | 2.56 ± 0.57 a | 3.50 ± 0.44 a | 26.7 ± 5.7 a | 1.7 ± 0.1 b | 521 ± 103 b |
| PG3 | 4.09 ± 0.98 b | 3.93 ± 0.97 b | 6.23 ± 1.36 b | 39.5 ± 5.1 b | 2.1 ± 0.3 a | 782 ± 91 c |
| PG4 | 6.16 ± 0.70 c | 6.04 ± 0.71 c | 13.49 ± 2.69 c | 50.7 ± 16.1 b,c | 3.5 ± 0.2 c | 488 ± 161 a,b |
| PG5 | 7.37 ± 1.82 c | 7.03 ± 1.71 c | 18.06 ± 5.29 d | 52.5 ± 11.4 c | 4.1 ± 0.6 d | 474 ± 188 a,b |
| Hydrogel | Saliva Parameters | Viscosity Parameters | |||||
|---|---|---|---|---|---|---|---|
| SU * (%) | SIR ** (g/min) | K (Pa × s) | R2 | n | ηapp 10 (s−1) | ηapp 50 (s−1) | |
| PG1 | 22.6 ± 14.0 a | 3.04 ± 2.6 a | 194.49 | 0.972 | −0.788 | 50.6 ± 22.1 a | 2.8 ± 1.1 a |
| PG3 | 24.0 ± 12.7 a | 3.42 ± 2.7 a | 181.39 | 0.962 | −0.778 | 47.2 ± 19.7 a | 2.9 ± 0.9 a |
| PG5 | 29.1 ± 13.4 b | 3.38 ± 2.2 a | 170.65 | 0.974 | −0.811 | 43.5 ± 25.7 a | 2.1 ± 0.7 a |
| Sensory Attributes 1 | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| General properties | |||||||
| SR | −0.26 | 0.31 * | −0.01 | −0.03 | 0.09 | −0.38 * | 0.10 |
| WL | −0.29 * | −0.01 | 0.21 | −0.22 | −0.11 | 0.01 | 0.07 |
| Rheological properties | |||||||
| G′LVE (Pa) | 0.17 | −0.30 * | 0.07 | −0.03 | −0.11 | 0.36 * | −0.07 |
| G″LVE (Pa) | 0.21 | −0.31 * | 0.05 | −0.01 | −0.11 | 0.37 * | −0.08 |
| γFR | −0.12 | 0.28 | −0.10 | 0.06 | 0.12 | −0.34 * | 0.06 |
| k′ (Pa*s) | 0.21 | −0.31 * | 0.05 | −0.01 | −0.11 | 0.37 * | −0.08 |
| k″ (Pa*s) | 0.23 | −0.31 * | 0.04 | 0.01 | −0.10 | 0.37 * | −0.09 |
| ƞ*s | 0.37 * | −0.29 * | −0.08 | 0.12 | −0.04 | 0.34 * | −0.12 |
| A | 0.29 * | −0.31 * | 0.00 | 0.05 | −0.08 | 0.37 * | −0.10 |
| Viscosity | |||||||
| ƞ10 | 0.34 * | −0.31 * | −0.04 | 0.09 | −0.06 | 0.36 * | −0.11 |
| ƞ55 | 0.31 * | −0.31 * | −0.02 | 0.06 | −0.07 | 0.37 * | −0.11 |
| Mechanical properties | |||||||
| Hardness | 0.26 | −0.31 * | 0.01 | 0.03 | −0.09 | 0.38 * | −0.10 |
| Fracturability | 0.27 | −0.31 * | 0.01 | 0.03 | −0.09 | 0.38 * | −0.10 |
| Brittleness | 0.31 * | −0.31 * | −0.02 | 0.06 | −0.07 | 0.37 * | −0.11 |
| Adhesiveness | 0.40 * | −0.23 | −0.14 | 0.18 | 0.01 | 0.27 | −0.12 |
| E | −0.40 * | 0.22 | 0.15 | −0.18 | −0.01 | −0.26 | 0.12 |
| Consistency | −0.29 * | −0.31 * | 0.00 | 0.04 | −0.08 | 0.37 * | −0.10 |
| Liking and Sensory Attributes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Overall liking | - | ||||||||||
| Consistency liking | 0.65 * | - | |||||||||
| Aroma | 0.03 | 0.04 | - | ||||||||
| Taste | 0.36 * | 0.22 | 0.35 * | - | |||||||
| Hardness | −0.07 | −0.22 | 0.08 | 0.08 | - | ||||||
| Brittleness | 0.22 | 0.39 * | 0.41 * | 0.50 * | −0.12 | - | |||||
| Moisture | 0.05 | 0.37 * | −0.14 | 0.17 | −0.32 * | 0.31 * | - | ||||
| Springiness | −0.16 | 0.12 | 0.43 * | 0.07 | 0.27 | 0.23 | 0.19 | - | |||
| Adhesiveness | 0.14 | −0.03 | 0.10 | 0.17 | −0.12 | 0.18 | 0.18 | −0.01 | - | ||
| Chewiness | −0.13 | −0.20 | −0.06 | −0.06 | 0.62 * | −0.20 | −0.27 | 0.05 | −0.10 | - | |
| Ease to swallow | 0.32 * | 0.43 * | 0.18 | 0.24 | −0.42 * | 0.28 | 0.33 * | −0.01 | 0.04 | −0.36 * | - |
| Sensory Attributes 1 | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Duration | −0.02 | −0.17 | −0.28 | −0.02 | −0.05 | 0.07 | −0.12 |
| Number of chews | −0.02 | −0.11 | −0.27 | −0.05 | 0.05 | 0.12 | −0.13 |
| MA (mV) | 0.23 | −0.04 | −0.17 | 0.27 | −0.05 | 0.31 * | −0.46 * |
| TA (mV) | 0.23 | −0.13 | −0.19 | 0.29 * | −0.13 | 0.17 | −0.55 * |
| MA (mV × s) | 0.12 | −0.10 | −0.23 | 0.12 | −0.06 | 0.23 | −0.38 * |
| TA (mV × s) | 0.12 | −0.17 | −0.28 | 0.13 | −0.13 | 0.20 | −0.42 * |
| Saliva uptake | 0.23 | −0.07 | −0.16 | 0.03 | 0.02 | 0.36 * | 0.08 |
| Saliva incorporation rate | 0.22 | −0.02 | −0.02 | 0.02 | 0.01 | 0.26 | −0.04 |
| Viscosity of bolus: | |||||||
| ƞ10 | −0.19 | 0.01 | 0.26 | −0.06 | 0.14 | −0.30 * | 0.12 |
| ƞ55 | −0.10 | −0.01 | 0.14 | −0.10 | 0.34 * | −0.20 | −0.06 |
| Pectin | Monosaccharides (mol%) a | Rha/GalA | RG-I% b | (Ara + Gal)/Rha c | DM d | Mw, kDa | Mw/Mn | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UA | Gal | Xyl | Glc | Rha | Ara | |||||||
| HP | 90.6 ± 0.7 | 3.2 ± 0.2 | 1.0 ± 0.3 | 0.5 ± 0.2 | 2.1 ± 0.1 | 3.3 ± 0.1 | 0.02 | 10.61 | 3.13 | 21 | 538 | 4.1 |
| AP | 89.5 ± 0.7 | 2.6 ± 2.6 | 3.8 ± 0.1 | 1.7 ± 0.1 | 1.6 ± 0.1 | 0.8 ± 0.5 | 0.02 | 6.61 | 2.14 | 43 | 401 | 5.2 |
| Hydrogel | AP (w/v %) | HP (w/v %) | Sucrose (w/v %) |
|---|---|---|---|
| PG1 | 4 | - | 10 |
| PG2 | 3 | 1 | 10 |
| PG3 | 2 | 2 | 10 |
| PG4 | 1 | 3 | 10 |
| PG5 | - | 4 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, S.; Smirnov, V.; Khramova, D.; Paderin, N.; Chistiakova, E.; Ptashkin, D.; Vityazev, F. Effect of Hogweed Pectin on Rheological, Mechanical, and Sensory Properties of Apple Pectin Hydrogel. Gels 2023, 9, 225. https://doi.org/10.3390/gels9030225
Popov S, Smirnov V, Khramova D, Paderin N, Chistiakova E, Ptashkin D, Vityazev F. Effect of Hogweed Pectin on Rheological, Mechanical, and Sensory Properties of Apple Pectin Hydrogel. Gels. 2023; 9(3):225. https://doi.org/10.3390/gels9030225
Chicago/Turabian StylePopov, Sergey, Vasily Smirnov, Daria Khramova, Nikita Paderin, Elizaveta Chistiakova, Dmitry Ptashkin, and Fedor Vityazev. 2023. "Effect of Hogweed Pectin on Rheological, Mechanical, and Sensory Properties of Apple Pectin Hydrogel" Gels 9, no. 3: 225. https://doi.org/10.3390/gels9030225
APA StylePopov, S., Smirnov, V., Khramova, D., Paderin, N., Chistiakova, E., Ptashkin, D., & Vityazev, F. (2023). Effect of Hogweed Pectin on Rheological, Mechanical, and Sensory Properties of Apple Pectin Hydrogel. Gels, 9(3), 225. https://doi.org/10.3390/gels9030225