Collagen Membrane as Water-Based Gel Electrolyte for Electrochromic Devices
Abstract
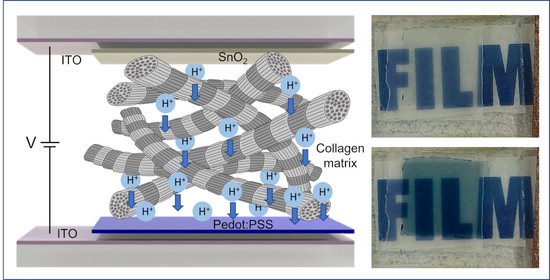
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Collagen Membranes and Collagen Electrolyte Preparation
4.3. Electrochromic Device Fabrication
4.4. Collagen Membrane and Collagen Electrolyte Characterization
4.5. Electrochromic Device Characterization
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeganeh Ghotbi, M. Solid state electrolytes for electrochemical energy devices. J. Mater. Sci. Mater. Electron. 2019, 30, 13835–13854. [Google Scholar] [CrossRef]
- Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C. A review of polymer electrolytes: Fundamental, approaches and applications. Ionics 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Ren, W.; Ding, C.; Fu, X.; Huang, Y. Advanced gel polymer electrolytes for safe and durable lithium metal batteries: Challenges, strategies, and perspectives. Energy Storage Mater. 2021, 34, 515–535. [Google Scholar] [CrossRef]
- Primiceri, V.; Pugliese, M.; Prontera, C.T.; Monteduro, A.G.; Esposito, M.; Maggiore, A.; Cannavale, A.; Giannuzzi, R.; Gigli, G.; Maiorano, V. Low-cost gel polymeric electrolytes for electrochromic applications. Sol. Energy Mater. Sol. Cells 2022, 240, 111657. [Google Scholar] [CrossRef]
- Yang, H.; Yang, J.; Li, C.; Huang, Z.; Bendavid, A.; Yan, J.; Cen, K.; Han, Z.; Bo, Z. Gel polymer dominated ion charging mechanisms within graphene nanochannels. J. Power Sources 2022, 541, 231684. [Google Scholar] [CrossRef]
- Choudhury, N.A.; Sampath, S.; Shukla, A.K. Hydrogel-polymer electrolytes for electrochemical capacitors: An overview. Energy Environ. Sci. 2009, 2, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J. Bio-based composite hydrogels for biomedical applications. Multifunct. Mater. 2020, 3, 22001. [Google Scholar] [CrossRef]
- Varshney, P.K.; Gupta, S. Natural polymer-based electrolytes for electrochemical devices: A review. Ionics 2011, 17, 479–483. [Google Scholar] [CrossRef]
- Rayung, M.; Aung, M.M.; Azhar, S.C.; Abdullah, L.C.; Su’ait, M.S.; Ahmad, A.; Jamil, S.N.A.M. Bio-based polymer electrolytes for electrochemical devices: Insight into the ionic conductivity performance. Materials 2020, 13, 838. [Google Scholar] [CrossRef] [Green Version]
- Huo, P.; Ni, S.; Hou, P.; Xun, Z.; Liu, Y.; Gu, J. A Crosslinked Soybean Protein Isolate Gel Polymer Electrolyte Based on Neutral Aqueous Electrolyte for a High-Energy-Density Supercapacitor. Polymers 2019, 11, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Railanmaa, A.; Kujala, M.; Keskinen, J.; Kololuoma, T.; Lupo, D. Highly flexible and non-toxic natural polymer gel electrolyte for printed supercapacitors for IoT. Appl. Phys. A 2019, 125, 168. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Wang, Y.; Liao, X.; Shi, B. A collagen-based electrolyte-locked separator enables capacitor to have high safety and ionic conductivity. J. Energy Chem. 2020, 47, 324–332. [Google Scholar] [CrossRef]
- Matsuo, Y.; Ikeda, H.; Kawabata, T.; Hatori, J.; Oyama, H. Collagen-Based Fuel Cell and Its Proton Transfer. Mater. Sci. Appl. 2017, 8, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Chandra, S.; Dwivedi, S.; Srivastava, A.; Ompj, P.V. Collagen: A Brief Analysis. Oral Maxillofac. Pathol. J. 2019, 10, 11–17. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Bella, J. Collagen structure: New tricks from a very old dog. Biochem. J. 2016, 473, 1001–1025. [Google Scholar] [CrossRef] [PubMed]
- Birk, D.E.; Brückner, P. Collagens, Suprastructures, and Collagen Fibril Assembly BT—The Extracellular Matrix: An Overview; Mecham, R.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 77–115. ISBN 978-3-642-16555-9. [Google Scholar]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Terzi, A.; Sannino, A.; Madaghiele, M. Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 644595. [Google Scholar] [CrossRef]
- Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L. An overview of the use of equine collagen as emerging material for biomedical applications. J. Funct. Biomater. 2020, 11, 79. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater. Sci. Eng. C 2019, 99, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhou, K.; Zhu, Y.; Zhang, W.; Xie, Y.; Wang, Z.; Zhou, H.; Yang, T.; Zhang, Q.; Xu, B. Collagen and its derivatives: From structure and properties to their applications in food industry. Food Hydrocoll. 2022, 131, 107748. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [Green Version]
- Noorzai, S.; Verbeek, C.J.R.; Lay, M.C.; Swan, J. Collagen Extraction from Various Waste Bovine Hide Sources. Waste Biomass Valorization 2020, 11, 5687–5698. [Google Scholar] [CrossRef]
- Noorzai, S.; Verbeek, C.J.R. Collagen: From Waste to Gold; Basso, T.P., Basso, T.O., Basso, L.C., Eds.; IntechOpen: Rijeka, Croatia, 2020; p. 12. ISBN 978-1-83881-182-2. [Google Scholar]
- Lionetto, F.; Bagheri, S.; Mele, C. Sustainable Materials from Fish Industry Waste for Electrochemical Energy Systems. Energies 2021, 14, 7928. [Google Scholar] [CrossRef]
- Harley, B.A.; Hastings, A.Z.; Yannas, I.V.; Sannino, A. Fabricating tubular scaffolds with a radial pore size gradient by a spinning technique. Biomaterials 2006, 27, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; De Caro, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and Supramolecular X-ray Characterization of Engineered Tissues from Equine Tendon, Bovine Dermis, and Fish Skin Type-I Collagen. Macromol. Biosci. 2020, 20, 2000017. [Google Scholar] [CrossRef]
- Gallo, N.; Natali, M.L.; Curci, C.; Picerno, A.; Gallone, A.; Vulpi, M.; Vitarelli, A.; Ditonno, P.; Cascione, M.; Sallustio, F.; et al. Analysis of the physico-chemical, mechanical and biological properties of crosslinked type-i collagen from horse tendon: Towards the development of ideal scaffolding material for urethral regeneration. Materials 2021, 14, 7648. [Google Scholar] [CrossRef]
- Bettini, S.; Bonfrate, V.; Syrgiannis, Z.; Sannino, A.; Salvatore, L.; Madaghiele, M.; Valli, L.; Giancane, G. Biocompatible Collagen Paramagnetic Scaffold for Controlled Drug Release. Biomacromolecules 2015, 16, 2599–2608. [Google Scholar] [CrossRef]
- Gallo, L.C.; Madaghiele, M.; Salvatore, L.; Barca, A.; Scialla, S.; Bettini, S.; Valli, L.; Verri, T.; Bucalá, V.; Sannino, A. Integration of PLGA Microparticles in Collagen-Based Matrices: Tunable Scaffold Properties and Interaction Between Microparticles and Human Epithelial-Like Cells. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 137–147. [Google Scholar] [CrossRef]
- Sanden, K.W.; Kohler, A.; Afseth, N.K.; Böcker, U.; Rønning, S.B.; Liland, K.H.; Pedersen, M.E. The use of Fourier-transform infrared spectroscopy to characterize connective tissue components in skeletal muscle of Atlantic cod (Gadus morhua L.). J. Biophotonics 2019, 12, e201800436. [Google Scholar] [CrossRef] [Green Version]
- De Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Nucara, A. Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region. Amino Acids 2010, 38, 679–690. [Google Scholar] [CrossRef]
- Sow, L.C.; Peh, Y.R.; Pekerti, B.N.; Fu, C.; Bansal, N.; Yang, H. Nanostructural analysis and textural modification of tilapia fish gelatin affected by gellan and calcium chloride addition. LWT Food Sci. Technol. 2017, 85, 137–145. [Google Scholar] [CrossRef]
- Shi, C.; Bi, C.; Ding, M.; Xie, J.; Xu, C.; Qiao, R.; Wang, X.; Zhong, J. Polymorphism and stability of nanostructures of three types of collagens from bovine flexor tendon, rat tail, and tilapia skin. Food Hydrocoll. 2019, 93, 253–260. [Google Scholar] [CrossRef]
- Yan, M.; Qin, S.; Li, J. Study on the self-assembly property of type I collagen prepared from tilapia (Oreochromis niloticus) skin by different extraction methods. Int. J. Food Sci. Technol. 2015, 50, 2088–2096. [Google Scholar] [CrossRef]
- Gallo, N.; Natali, M.L.; Quarta, A.; Gaballo, A.; Terzi, A.; Sibillano, T.; Giannini, C.; De Benedetto, G.E.; Lunetti, P.; Capobianco, L.; et al. Aquaponics-Derived Tilapia Skin Collagen for Biomaterials Development. Polymers 2022, 14, 1865. [Google Scholar] [CrossRef]
- Bridelli, M.G. Fourier Transform Infrared Spectroscopy in the Study of Hydrated Biological Macromolecules; Nikolic, G.S., Cakic, M.D., Cvetkovic, D.J., Eds.; IntechOpen: Rijeka, Croatia, 2017; p. 9. ISBN 978-953-51-2894-6. [Google Scholar]
- Rath, A.; Davidson, A.R.; Deber, C.M. The structure of “unstructured” regions in peptides and proteins: Role of the polyproline II helix in protein folding and recognition. Pept. Sci. 2005, 80, 179–185. [Google Scholar] [CrossRef]
- Demeter, M.; Călina, I.; Scărișoreanu, A.; Micutz, M.; Kaya, M.A. Correlations on the Structure and Properties of Collagen Hydrogels Produced by E-Beam Crosslinking. Materials 2022, 15, 7663. [Google Scholar] [CrossRef]
- Nakamoto, K.; Margoshes, M.; Rundle, R.E. Stretching Frequencies as a Function of Distances in Hydrogen Bonds. J. Am. Chem. Soc. 1955, 77, 6480–6486. [Google Scholar] [CrossRef]
- Bridelli, M.G.; Stani, C.; Bedotti, R. Fourier transform infrared conformational investigation of type I collagen aged by in vitro induced dehydration and non-enzymatic glycation treatments. J. Biol. Res. Boll. Soc. Ital. Biol. Sper. 2017, 90. [Google Scholar] [CrossRef]
- Bridelli, M.G.; Crippa, P.R. Infrared and Water Sorption Studies of the Hydration Structure and Mechanism in Natural and Synthetic Melanin. J. Phys. Chem. B 2010, 114, 9381–9390. [Google Scholar] [CrossRef] [PubMed]
- Sideroudi, H.; Labiris, G.; Soto-Beobide, A.; Voyiatzis, G.; Chrissanthopoulos, A.; Kozobolis, V. The Effect of Collagen Cross-Linking Procedure on the Material of Intracorneal Ring Segments. Curr. Eye Res. 2015, 40, 592–597. [Google Scholar] [CrossRef]
- Matsui, H.; Matsuo, Y. Proton Conduction via Water Bridges Hydrated in the Collagen Film. J. Funct. Biomater. 2020, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Graßmann, C.; Mann, M.; Van Langenhove, L.; Schwarz-Pfeiffer, A. Textile Based Electrochromic Cells Prepared with PEDOT: PSS and Gelled Electrolyte. Sensors 2020, 20, 5691. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Aiello, D.; Lunetti, P.; Barca, A.; Blasi, L.; Madaghiele, M.; Bettini, S.; Giancane, G.; Hasan, M.; et al. An insight on type I collagen from horse tendon for the manufacture of implantable devices. Int. J. Biol. Macromol. 2020, 154, 291–306. [Google Scholar] [CrossRef]








| Sample | β-Sheets 1610–1642 cm−1 | Random Coil 1642–1650 cm−1 | α-Helices 1650–1660 cm−1 | β-Turn 1660–1680 cm−1 | β-Antiparallel 1680–1700 cm−1 |
|---|---|---|---|---|---|
| UN film | 1626 (29.8%) | 1645 (32.1%) | 1660 (26.0%) | 1679 (7.4%) | 1695 (4.7%) |
| DHT film | 1625 (45.8%) | 1638 (1.6%) | 1655 (46.6%) | 1681.9 (4.1%) | 1694.4 (1.9%) |
| Stretching Frequency (1/cm) | ν1 | ν2 | ν3 | ν4 |
|---|---|---|---|---|
| H-bond distances (nm) | 0.31 | 0.29 | 0.28 | 0.25 |
| UN film | 3673 (4.4%) | 3490 (18.6%) | 3250 (50.6%) | 3116 (26.4%) |
| DHT film | 3671 (0.0%) | 3482 (16.8%) | 3249 (50.0%) | 3135 (33.2%) |
| Sample Type | E (MPa) | 𝛔max (MPa) | 𝛆r% (-) |
|---|---|---|---|
| Uncosslinked collagen membrane (H2O) | 1.2 ± 0.3 | 1.8 ± 0.6 | 131 ± 7 |
| Uncosslinked collagen membrane (1% H2SO4) | - | - | - |
| Uncosslinked collagen membrane (2% H2SO4) | - | - | - |
| Uncosslinked collagen membrane (5% H2SO4) | - | - | - |
| Cross-linked collagen membrane (H2O) | 2.7 ± 0.5 | 2.3 ± 0.3 | 67 ± 7 |
| Cross-linked collagen membrane (1% H2SO4) | 4.7 ± 0.6 | 2.3 ± 0.4 | 40 ± 8 |
| Cross-linked collagen membrane (2% H2SO4) | 2.8 ± 0.5 | 2.7 ± 1.2 | 65 ± 15 |
| Cross-linked collagen membrane (5% H2SO4) | 2.5 ± 0.1 | 1.5 ± 0.4 | 55 ± 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prontera, C.T.; Gallo, N.; Giannuzzi, R.; Pugliese, M.; Primiceri, V.; Mariano, F.; Maggiore, A.; Gigli, G.; Sannino, A.; Salvatore, L.; et al. Collagen Membrane as Water-Based Gel Electrolyte for Electrochromic Devices. Gels 2023, 9, 310. https://doi.org/10.3390/gels9040310
Prontera CT, Gallo N, Giannuzzi R, Pugliese M, Primiceri V, Mariano F, Maggiore A, Gigli G, Sannino A, Salvatore L, et al. Collagen Membrane as Water-Based Gel Electrolyte for Electrochromic Devices. Gels. 2023; 9(4):310. https://doi.org/10.3390/gels9040310
Chicago/Turabian StyleProntera, Carmela Tania, Nunzia Gallo, Roberto Giannuzzi, Marco Pugliese, Vitantonio Primiceri, Fabrizio Mariano, Antonio Maggiore, Giuseppe Gigli, Alessandro Sannino, Luca Salvatore, and et al. 2023. "Collagen Membrane as Water-Based Gel Electrolyte for Electrochromic Devices" Gels 9, no. 4: 310. https://doi.org/10.3390/gels9040310
APA StyleProntera, C. T., Gallo, N., Giannuzzi, R., Pugliese, M., Primiceri, V., Mariano, F., Maggiore, A., Gigli, G., Sannino, A., Salvatore, L., & Maiorano, V. (2023). Collagen Membrane as Water-Based Gel Electrolyte for Electrochromic Devices. Gels, 9(4), 310. https://doi.org/10.3390/gels9040310