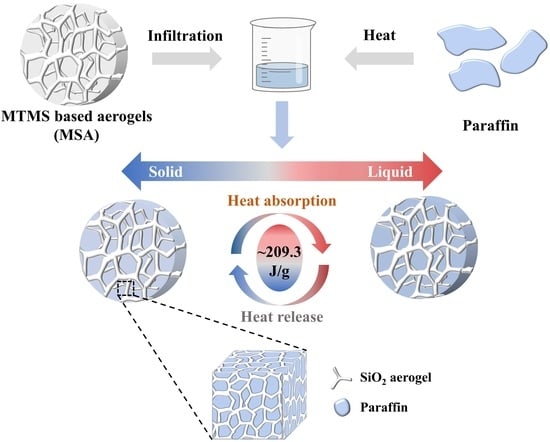
Encapsulation of Paraffin Phase-Change Materials within Monolithic MTMS-Based Silica Aerogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Basic Characterization of the Paraffin/MSA Composites
2.2. Microstructure of the Paraffin/MSA Composites
2.3. Mechanical Property of the Paraffin/MSA Composites
2.4. Hydrophobicity and FTIR of the Paraffin/MSA Composites
2.5. Thermal Analysis of the Paraffin/MSA Composites
2.6. Comparison between Aerogel-Based Phase-Change Materials
3. Conclusions
4. Experimental Section
4.1. Raw Materials
4.2. Sample Preparation
4.3. Methods of Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Memon, S.A. Phase Change Materials Integrated in Building Walls: A State of the Art Review. Renew. Sustain. Energy Rev. 2014, 31, 870–906. [Google Scholar] [CrossRef]
- Wang, A.; Hu, M.; Tao, D.; Wang, J. Analysis and Application of Phase Change Materials on Energy Saving in Buildings. Adv. Mater. Res. 2011, 411, 523–526. [Google Scholar] [CrossRef]
- Jia, W.; Wang, C.; Wang, T.; Cai, Z.; Chen, K. Preparation and Performances of Palmitic Acid/Diatomite Form-stable Composite Phase Change Materials. Int. J. Energy Res. 2020, 44, 4298–4308. [Google Scholar] [CrossRef]
- Schossig, P.; Henning, H.; Gschwander, S.; Haussmann, T. Micro-Encapsulated Phase-Change Materials Integrated into Construction Materials. Sol. Energy Mater. Sol. Cells 2005, 89, 297–306. [Google Scholar] [CrossRef]
- Lei, C.; Wu, K.; Wu, L.; Liu, W.; Du, R.; Chen, F.; Fu, Q. Phase Change Material with Anisotropically High Thermal Conductivity and Excellent Shape Stability due to Its Robust Cellulose/BNNSs Skeleton. J. Mater. Chem. A 2019, 7, 19364–19373. [Google Scholar] [CrossRef]
- Pulci, G.; Paglia, L.; Genova, V.; Bartuli, C.; Valente, T.; Marra, F. Low Density Ablative Materials Modified by Nanoparticles Addition: Manufacturing and Characterization. Compos. Part A Appl. Sci. Manuf. 2018, 109, 330–337. [Google Scholar] [CrossRef]
- Cui, Y.; Xie, J.; Liu, J.; Wang, J.; Chen, S. A Review on Phase Change Material Application in Building. Adv. Mech. Eng. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Fang, G.; Tang, F.; Cao, L. Preparation, Thermal Properties and Applications of Shape-Stabilized Thermal Energy Storage Materials. Renew. Sustain. Energy Rev. 2014, 40, 237–259. [Google Scholar] [CrossRef]
- Yan, Q.Y.; Jin, L.L.; Zhang, L. A Study on the Development of Phase Change Material Wall. AMR Adv. Mater. Res. 2011, 347–353, 2773–2776. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal Stability of Phase Change Materials Used in Latent Heat Energy Storage Systems: A Review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- He, B.; Martin, V.; Setterwall, F. Phase Transition Temperature Ranges and Storage Density of Paraffin Wax Phase Change Materials. Energy 2004, 29, 1785–1804. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, N.; Wang, H.; Sun, R.; Wong, C.-P. A Newly Designed Paraffin@VO2 Phase Change Material with the Combination of High Latent Heat and Large Thermal Conductivity. J. Colloid Interface Sci. 2020, 559, 226–235. [Google Scholar] [CrossRef]
- Mishra, D.K.; Bhowmik, C.; Bhowmik, S.; Pandey, K.M. Property-Enhanced Paraffin-Based Composite Phase Change Material for Thermal Energy Storage: A Review. Environ. Sci. Pollut. Res. 2022, 29, 43556–43587. [Google Scholar] [CrossRef] [PubMed]
- França, D.; Pereira, V.B.; Coutinho, D.M.; Ainstein, L.M.; Azevedo, D.A. Speciation and Quantification of High Molecular Weight Paraffins in Brazilian Whole Crude Oils Using High-Temperature Comprehensive Two-Dimensional Gas Chromatography. Fuel 2018, 234, 1154–1164. [Google Scholar] [CrossRef]
- Xiao, M.; Feng, B.; Gong, K. Preparation and Performance of Shape Stabilized Phase Change Thermal Storage Materials with High Thermal Conductivity. Energy Convers. Manag. 2002, 43, 103–108. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, Methods, Techniques, and Applications for Microencapsulated Phase Change Materials (MPCM): A Review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Cao, L.; Zhang, D. Composite Phase Change Material Based on Reduced Graphene Oxide/Expanded Graphite Aerogel with Improved Thermal Properties and Shape-Stability. Int. J. Energy Res. 2020, 44, 242–256. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Review on Clay Mineral-Based Form-Stable Phase Change Materials: Preparation, Characterization and Applications. Renew. Sustain. Energy Rev. 2017, 68, 707–726. [Google Scholar] [CrossRef]
- Şahan, N.; Paksoy, H. Investigating Thermal Properties of Using Nano-Tubular ZnO Powder in Paraffin as Phase Change Material Composite for Thermal Energy Storage. Compos. Part B Eng. 2017, 126, 88–93. [Google Scholar] [CrossRef]
- Song, M.; Jiang, J.; Zhu, J.; Zheng, Y.; Yu, Z.; Ren, X.; Jiang, F. Lightweight, Strong, and Form-Stable Cellulose Nanofibrils Phase Change Aerogel with High Latent Heat. Carbohydr. Polym. 2021, 272, 118460. [Google Scholar] [CrossRef] [PubMed]
- Konuklu, Y.; Ersoy, O. Preparation and Characterization of Sepiolite-Based Phase Change Material Nanocomposites for Thermal Energy Storage. Appl. Therm. Eng. 2016, 107, 575–582. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, D. Application Potential of Graphene Aerogel in Paraffin Phase Change Composites: Experimental Study and Guidance Based on Numerical Simulation. Sol. Energy Mater. Sol. Cells 2021, 223, 110949. [Google Scholar] [CrossRef]
- He, S.; Huang, Y.; Chen, G.; Feng, M.; Dai, H.; Yuan, B.; Chen, X. Effect of Heat Treatment on Hydrophobic Silica Aerogel. J. Hazard. Mater. 2019, 362, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Koebel, M.M.; Malfait, W.J. Silica Aerogels with Tailored Chemical Functionality. Mater. Des. 2020, 193, 108833. [Google Scholar] [CrossRef]
- Mazrouei-Sebdani, Z.; Begum, H.; Schoenwald, S.; Horoshenkov, K.V.; Malfait, W.J. A Review on Silica Aerogel-Based Materials for Acoustic Applications. J. Non-Cryst. Solids 2021, 562, 120770. [Google Scholar] [CrossRef]
- Hasan, M.A.; Rashmi, S.; Esther, A.C.M.; Bhavanisankar, P.Y.; Sherikar, B.N.; Sridhara, N.; Dey, A. Evaluations of Silica Aerogel-Based Flexible Blanket as Passive Thermal Control Element for Spacecraft Applications. J. Mater. Eng. Perform. 2018, 27, 1265–1273. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward Aerogel Based Thermal Superinsulation in Buildings: A Comprehensive Review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Liu, P.; Chen, X.; Li, Y.; Cheng, P.; Tang, Z.; Lv, J.; Aftab, W.; Wang, G. Aerogels Meet Phase Change Materials: Fundamentals, Advances, and Beyond. ACS Nano 2022, 16, 15586–15626. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, H.; Feng, J.; Zhang, C.; Jiang, Y. Preparation and Thermal Properties of Paraffin/Porous Silica Ceramic Composite. Compos. Sci. Technol. 2009, 69, 1246–1249. [Google Scholar] [CrossRef]
- Li, H.; Chen, H.; Li, X.; Sanjayan, J.G. Development of Thermal Energy Storage Composites and Prevention of PCM Leakage. Appl. Energy 2014, 135, 225–233. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, J.; Wang, G.; Zhang, R.; Peng, X. Preparation of Paraffin/SiO2 Aerogel Stable-Stabilized Phase Change Composites for High-Humidity Environment. J. Mater. Sci. 2020, 55, 1511–1524. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Huang, S.; Wu, X.; Shi, L.; Liu, Q. Thermal Stability and Pyrolysis Characteristics of MTMS Aerogels Prepared in Pure Water. J. Nanopart. Res. 2020, 22, 334. [Google Scholar] [CrossRef]
- Wang, X.; Deng, X.; Wu, L.; Deng, Y.; Liu, Q.; Li, M.; Li, Z. Facile Preparation of Mechanically Strong Polyvinyl Alcohol/MTMS Aerogel Composites with Improved Thermal Stability. J. Nanopart. Res. 2021, 23, 261. [Google Scholar] [CrossRef]
- Huang, S.; Wu, X.; Li, Z.; Shi, L.; Zhang, Y.; Liu, Q. Rapid Synthesis and Characterization of Monolithic Ambient Pressure Dried MTMS Aerogels in Pure Water. J. Porous Mater. 2020, 27, 1241–1251. [Google Scholar] [CrossRef]
- Wu, X.; Huang, S.; Zhang, Y.; Shi, L.; Luo, Y.; Deng, X.; Liu, Q.; Li, Z. Flame Retardant Polyurethane Sponge/MTMS Aerogel Composites with Improved Mechanical Properties under Ambient Pressure Drying. J. Nanopart. Res. 2020, 22, 221. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Zhang, W.; Yan, H.; Wang, Y.; Li, M.; Liu, Q. Rapid Synthesis and Characterization of Ambient Pressure Dried Monolithic Silica Aerogels in Ethanol/Water Co-Solvent System. J. Non-Cryst. Solids 2019, 503–504, 214–223. [Google Scholar] [CrossRef]
- M., H.; Gopakumar, D.; Arumughan, V.; Pottathara, Y.; K. S., S.; Pasquini, D.; Bračič, M.; Seantier, B.; Nzihou, A.; Thomas, S.; et al. Robust Superhydrophobic Cellulose Nanofiber Aerogel for Multifunctional Environmental Applications. Polymers 2019, 11, 495. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Wu, L.; Deng, Y.; Huang, S.; Sun, M.; Wang, X.; Liu, Q.; Li, M.; Li, Z. Effects of Precursor Concentration on the Physicochemical Properties of Ambient-Pressure-Dried MTES Based Aerogels with Using Pure Water as the Only Solvent. J. Sol-Gel Sci. Technol. 2021, 100, 477–488. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and Characterization by FTIR Spectroscopy of Silica Aerogels Prepared Using Several Si(OR)4 and R′′Si(OR′)3 Precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Stein, R.S.; Sutherland, G.B.B.M. Effect of Intermolecular Interactions between CH Frequencies on the Infrared Spectra of N -Paraffins and Polythene. J. Chem. Phys. 1954, 22, 1993–1999. [Google Scholar] [CrossRef] [Green Version]
- Chomel, A.D.; Dempsey, P.; Latournerie, J.; Hourlier-Bahloul, D.; Jayasooriya, U.A. Gel to Glass Transformation of Methyltriethoxysilane: A Silicon Oxycarbide Glass Precursor Investigated Using Vibrational Spectroscopy. Chem. Mater. 2005, 17, 4468–4473. [Google Scholar] [CrossRef]
- Gurmen Ozcelik, T. Preparation, Characterization and Thermal Properties of Paraffin Wax–Expanded Perlite Form-Stable Composites for Latent Heat Storage. Mater. Sci. 2017, 23, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Evola, G.; Marletta, L.; Sicurella, F. A Methodology for Investigating the Effectiveness of PCM Wallboards for Summer Thermal Comfort in Buildings. Build. Environ. 2013, 59, 517–527. [Google Scholar] [CrossRef]
- Wang, C.; Feng, L.; Li, W.; Zheng, J.; Tian, W.; Li, X. Shape-Stabilized Phase Change Materials Based on Polyethylene Glycol/Porous Carbon Composite: The Influence of the Pore Structure of the Carbon Materials. Sol. Energy Mater. Sol. Cells 2012, 105, 21–26. [Google Scholar] [CrossRef]
- Li, M. A Nano-Graphite/Paraffin Phase Change Material with High Thermal Conductivity. Appl. Energy 2013, 106, 25–30. [Google Scholar] [CrossRef]
- Xiangfa, Z.; Hanning, X.; Jian, F.; Changrui, Z.; Yonggang, J. Preparation, Properties and Thermal Control Applications of Silica Aerogel Infiltrated with Solid-Liquid Phase Change Materials. J. Exp. Nanosci. 2012, 7, 17–26. [Google Scholar] [CrossRef]
- Gao, H.; Bo, L.; Liu, P.; Chen, D.; Li, A.; Ou, Y.; Dong, C.; Wang, J.; Chen, X.; Hou, C.; et al. Ambient Pressure Dried Flexible Silica Aerogel for Construction of Monolithic Shape-Stabilized Phase Change Materials. Sol. Energy Mater. Sol. Cells 2019, 201, 110122. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Deng, X.; Deng, Y.; Wu, X.; Shi, L.; Li, M.; Liu, Q.; Cheng, X.; Li, Z. Improving the Flame Retardance of Hydrophobic Silica Aerogels through a Facile Post-Doping of Magnesium Hydroxide. Adv. Powder Technol. 2021, 32, 1891–1901. [Google Scholar] [CrossRef]
- Sun, M.; Li, Z.; Zhang, Y.; Wu, X.; Shi, L.; Liu, Q.; Li, M. Assessment on Thermal Safety of Aluminum Hydroxide Doping Hydrophobic Silica Aerogels. J. Nanopart. Res. 2022, 24, 87. [Google Scholar] [CrossRef]
- Xiangfa, Z.; Hanning, X.; Jian, F.; Changrui, Z.; Yonggang, J. Pore Structure Modification of Silica Matrix Infiltrated with Paraffin as Phase Change Material. Chem. Eng. Res. Des. 2010, 88, 1013–1017. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]









| Physicochemical Property | MSA | Paraffin | Paraffin/MSA Composites |
|---|---|---|---|
| Density (g/cm3) | 0.074 | 0.82 | 0.70 |
| Thermal conductivity (mW/m/K) | 30.9 | 246.3 | 253.1 |
| Parameters | Paraffin in the Composites (<80 °C) | Paraffin | Paraffin/MSA Composites | MSA |
|---|---|---|---|---|
| I-Tonset (°C) | 27.02 | 31.87 | 205.72 | - |
| I-Tpeak (°C) | 40.95 | 45.21 | 266.17 | - |
| II-Tonset (°C) | 59.68 | 58.17 | 414.75 | 452.66 |
| II-Tpeak (°C) | 68.48 | 64.81 | 525.82 | 519.03 |
| Properties | Paraffin/MSA | Paraffin/TSA [30,47,51] |
|---|---|---|
| shape of aerogels | monolithic | powdery |
| solvent for aerogel preparation | pure water | multiple organic solvents |
| preparation time (h) | ~12 | >72 |
| pore size of aerogels | micron- | nano- |
| mass ratio of paraffin to aerogels | >8 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Wu, X.; Wang, G.; Shulga, Y.M.; Liu, Q.; Li, M.; Li, Z. Encapsulation of Paraffin Phase-Change Materials within Monolithic MTMS-Based Silica Aerogels. Gels 2023, 9, 317. https://doi.org/10.3390/gels9040317
Xie L, Wu X, Wang G, Shulga YM, Liu Q, Li M, Li Z. Encapsulation of Paraffin Phase-Change Materials within Monolithic MTMS-Based Silica Aerogels. Gels. 2023; 9(4):317. https://doi.org/10.3390/gels9040317
Chicago/Turabian StyleXie, Linlin, Xiaoxu Wu, Guichao Wang, Yury M. Shulga, Qiong Liu, Ming Li, and Zhi Li. 2023. "Encapsulation of Paraffin Phase-Change Materials within Monolithic MTMS-Based Silica Aerogels" Gels 9, no. 4: 317. https://doi.org/10.3390/gels9040317
APA StyleXie, L., Wu, X., Wang, G., Shulga, Y. M., Liu, Q., Li, M., & Li, Z. (2023). Encapsulation of Paraffin Phase-Change Materials within Monolithic MTMS-Based Silica Aerogels. Gels, 9(4), 317. https://doi.org/10.3390/gels9040317