Sodium Salt of Partially Carboxymethylated Sodium Alginate-g-Poly(acrylonitrile): I. Photo-Induced Synthesis, Characterization, and Alkaline Hydrolysis
Abstract
:1. Introduction
2. Results and Discussion
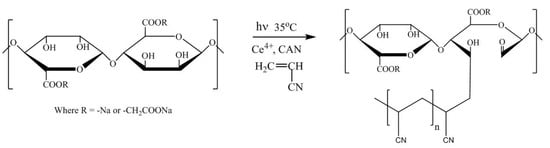
2.1. Synthesis of Na-PCMSA-g-PAN
2.2. Influence of the Polymerization Variables on the Grafting Yields
2.2.1. Calculation of Grafting Yields
2.2.2. Influence of Polymerization Variables
The Effect of Na-PCMSA Amount
The Photo-Initiator Concentration
The Acid (HNO3) Concentration
The Monomer Concentration
The Reaction Time
The Reaction Temperature
2.3. Saponification or Alkaline Hydrolysis
2.4. Characterization
2.4.1. FTIR Spectroscopy
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Scanning Electron Microscopy (SEM) Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Sodium Salt of Partially Carboxymethylated Sodium Alginate (Na–PCMSA)
4.2.2. Photo-Initiated Synthesis of Poly(acrylonitrile) Grafted Na–PCMSA (Na–PCMSA–g–PAN)
4.2.3. Purification of the Graft Copolymer by Solvent Extraction Method
4.3. Isolation of Grafted Chains
4.4. Alkaline Hydrolysis
4.5. Characterization Methods
4.5.1. FTIR Spectroscopy
4.5.2. Thermogravimetric Analysis (TGA)
4.5.3. Scanning Electron Microscopy (SEM)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattacharya, A.; Mishra, B.N. Grafting: A Versatile Means To Modify Polymers: Techniques, Factors and Applications. Pro. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Setia, A. Chapter 1—Applications of Graft Copolymerization: A Revolutionary Approach. In Biopolymer Grafting Applications, 1st ed.; Thakur, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–44. [Google Scholar]
- Kumar, D.; Pandey, J.; Raj, Y.; Kumar, P. A Review on the modification of polysaccharide through graft copolymerization for various potential applications. Open. Med. Chem. J. 2017, 11, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Hebeish, A.; Guthrie, J.T. The Chemistry And Technology of Cellulosic Copolymers; Springer-Veriag: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- El-Sherbiny, I.M.; Smyth, H.D.C. Photo-induced synthesis, characterization and swelling behavior of poly (2-hydroxyethyl methacrylate) grafted carboxymethyl chitosan. Carbohydr. Polym. 2010, 81, 652–659. [Google Scholar] [CrossRef]
- Grajek, K.; Andrzejewska, E. Recent advances in photo-induced free radical polymerization. MOJ Poly. Sci. 2017, 1, 58–60. [Google Scholar]
- Zongrui, T.; Yu, C.; Wei, L. Grafting Derivative from Alginate. In Biopolymer Grafting: Synthesis and Properties; Thakur, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 115–173. [Google Scholar]
- Basan, H.; Guruderelioglu, M.; Orbey, T. Diclofenac sodium releasing pH-sensitive monolithic devices. Int. J. Pharm. 2002, 245, 191–198. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Naik, H.G. Synthesis of graft copolymeric membranes of poly (vinyl alcohol) and polyacrylamide for the pervaporation separation of water/acetic acid mixtures. J. Appl. Polym. Sci. 2002, 83, 244–258. [Google Scholar] [CrossRef]
- Rousseau, I.; LeCerf, D.; Picton, L.; Argillier, J.F.; Muller, G. Entrapment and release of sodium polystyrene sulfonate(SPS)from calcium alginate gel beads. Eur. Polym. J. 2004, 40, 2709–2715. [Google Scholar] [CrossRef]
- Shah, S.B.; Patel, C.P.; Trivedi, H.C. Ceric-induced grafting of ethyl-acrylate onto sodium alginate. Die Angew. Makromol. Chemie 1994, 214, 75–89. [Google Scholar] [CrossRef]
- Shah, S.B.; Patel, C.P.; Trivedi, H.C. Ceric-induced grafting of acrylate monomers onto sodium alginate. Carbohydr. Polym. 1995, 26, 61–67. [Google Scholar] [CrossRef]
- Shah, S.B.; Patel, C.P.; Trivedi, H.C. Thermal behaviour of graft copolymers of sodium alginate. Die Angew. Makromol. Chemie 1996, 235, 1–13. [Google Scholar] [CrossRef]
- Radhakrishnan, N.; Lakshminarayana, Y.; Uma Devi, S.; Srinivasan, K.S.V. Studies on the Graft Copolymerization of Acrylonitrile onto Sodium Alginate. J. Macromol. Sci. Part A Pure Appl. Chem. 1994, 31, 581–591. [Google Scholar] [CrossRef]
- Akın, A.; Işıklan, N. Microwaveassistedsynthesisandcharacterizationofsodiumalginate-graft-poly(N,N′-dimethylacryla-mide). Int. J. Biol. Macromol. 2016, 82, 530–540. [Google Scholar]
- Liu, Y.; Yang, L.; Li, J.; Shi, Z. Grafting of methyl methacrylate onto sodium alginate initiated by potassium diperiodatocuprate (III). J. Appl. Polym. Sci. 2005, 97, 1688–1694. [Google Scholar] [CrossRef]
- Sen, G.; Singh, R.P.; Pal, S. Microwave-initiated synthesis of polyacrylonitrile grafted sodium alginate: Synthesis and characterization. J. Appl. Polym. Sci. 2010, 115, 63–71. [Google Scholar] [CrossRef]
- Salisu, A.; Sanagi, M.M.; Abu Naim, A.; Abd Karim, K.J.; Wan Ibrahim, W.A.; Abdulganiyu, U. Alginate graft polyacrylonitrile beads for the removal of lead from aqueous solutions. Polym. Bull. 2016, 73, 519–537. [Google Scholar] [CrossRef]
- Ciocoiu, O.-N.; Staikos, G.; Vasile, C. Thermoresponsive behavior of sodium alginate grafted with poly(N-isopropylacrylamide) in aqueous media. Carbohydr. Polym. 2018, 184, 118–126. [Google Scholar] [CrossRef]
- Da Feira, J.M.C.; Klein, J.M.; Forte, M.M.d.C. Ultrasound-assisted synthesis of polyacrylamide-grafted sodium alginate and its application in dye removal. Polímeros 2018, 28, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Flores-Hernández, C.G.; Cornejo-Villegas, M.d.L.A.; Moreno-Martell, A.; Real, A.D. Synthesis of a Biodegradable Polymer of Poly (Sodium Alginate/Ethyl Acrylate). Polymers 2021, 13, 504. [Google Scholar] [CrossRef]
- Sun, F.; Guo, J.; Liu, Y.; Yu, Y. Preparation, characterizations and properties of sodium alginate grafted acrylonitrile/polyethylene glycol electrospun nanofibers. Int. J. Biol. Macromol. 2019, 137, 420–425. [Google Scholar] [CrossRef]
- Trivedi, J.H.; Chourasia, A.V.; Trivedi, H.C. Photo-Induced Synthesis and Characterization of Poly(Methyl Acrylate) Grafted Sodium salt of Partially Carboxymethylated Sodium Alginate. Cellul. Chem. Technol. 2015, 49, 7–19. [Google Scholar]
- Athawale, V.D.; Rathi, S.C. Role and Relevance of Polarity and Solubility of Vinyl Monomers in Graft Polymerization onto Starch. React. Funct. Polym. 1997, 34, 11–17. [Google Scholar] [CrossRef]
- Athawale, V.D.; Rathi, S.C. Graft polymerization: Starch as a model substrate. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 1999, C39, 445–480. [Google Scholar] [CrossRef]
- Trivedi, J.H.; Chourasia, A.V. Sodium salt of Partially Carboxymethylated Sodium Alginate-graft-Poly(acrylonitrile): II Superabsorbency, Salt Sensitivity, and Swelling Kinetics Hydrogel, H-Na-PCMSA-g-PAN. Gels, 2023; in press. [Google Scholar]
- Goyal, P.; Kumar, V.; Sharma, P. Graft Copolymerization of Acrylamide onto Tamarind Kernel Powder in the Presence of Ceric ion. J. Appl. Polym. Sci. 2008, 108, 3696–3701. [Google Scholar] [CrossRef]
- Trivedi, J.H.; Thaker, M.D.; Trivedi, H.C. Photo-Induced Synthesis and Characterization of Poly (methyl methacrylate) grafted sodium salt of Partially Carboxymethylated Guar Gum. Chin. J. Polym. Sci. 2014, 32, 1690–1703. [Google Scholar] [CrossRef]
- Keles, H.; Sacak, M. Graft copolymerization of methyl methacrylate onto gelatin using KMnO4-H2SO4redox system. J. Appl. Polym. Sci. 2003, 89, 2836–2844. [Google Scholar] [CrossRef]
- Shi, Z.; Jia, C.; Wang, D.; Deng, J.; Xu, G.; Wu, C.; Dong, M.; Guo, Z. Synthesis and characterization of porous tree gum grafted copolymer derived from cerasifera gum polysaccharide. Int. J. Biol. Macromol. 2019, 133, 964–970. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Naushad, M.; Al-Misned, F.A.; El-Serehy, H.A.; Ghfar, A.A.; Sharma, K.R.; Si, C.; Stadler, F. Graft Copolymerization of Acrylonitrile and Ethyl Acrylate onto Pinus Roxburghii Wood Surface Enhanced Physicochemical Properties and Antibacterial Activity. J. Chem. 2020, 2020, 6285354. [Google Scholar] [CrossRef]
- Masry, B.A.; Shahr El-Din, A.M.; Al-Aidy, H.A. Ceric-ions redox initiating technique for Zirconium and Niobium separation through graft copolymerization of natural polysaccharides. Sep. Sci. Technol. 2022, 57, 603–618. [Google Scholar] [CrossRef]
- Patel, G.M.; Patel, C.P.; Trivedi, H.C. Ceric-induced grafting of methyl acrylate onto sodium salt of partially carboxymethylated sodium alginate. Eur. Polym. J. 1999, 35, 201–208. [Google Scholar] [CrossRef]
- Sharma, P.; Gupta, S.; Soni, P.L.; Kumar, V. Ce(IV)-ion initiated grafting of 1,3 galactomannan biopolymer with acrylonitrile. J. Macromol. Sci.-Pure Appl. Chem. 2020, 57, 519–530. [Google Scholar] [CrossRef]
- Kaur, I.; Kumar, R.; Sharma, N. A comparative study of the graft copolymerization acrylic acid onto rayon fiber by a ceric ion redox system and γ-radiation method. Carbohydr. Res. 2010, 345, 2164–2173. [Google Scholar] [CrossRef]
- Vora, R.A.; Trivedi, H.C.; Patel, C.P.; Trivedi, M.C. Grafting of Acrylonitrile onto Styrene-Maleic Acid Copolymer by Tetravalent Cerium Ion. J. Appl. Polym. Sci. 1995, 38, 1543–1550. [Google Scholar] [CrossRef]
- Trivedi, J.H.; Joshi, H.A.; Trivedi, H.C. Synthesis and Characterization of Photo-Graft Copolymers of Sodium salt of Partially Carboxymethylated Tamarind Kernel Powder. Marcomol. Symp. 2021, 398, 2000014. [Google Scholar] [CrossRef]
- Mukherjee, A.; Halder, S.; Datta, D.; Anupam, K.; Hazra, B.; Mandal, M.K.; Gopinath, H. Free radical induced grafting of acrylonitrile on pre-treated rice straw for enhancing its durability and flame retardancy. J. Adv. Res. 2017, 8, 73–83. [Google Scholar] [CrossRef]
- Taskin, G.; Sanli, O.; Asman, G. Swelling assisted photo grafting acid onto sodium alginate membranes. Appl. Surf. Sci. 2011, 257, 9444–9450. [Google Scholar] [CrossRef]
- Fanta, G.F.; Burr, R.C.; Doane, W.M.; Russel, C.R. Absorbent Polymers from Starch and Flour Through Graft Polymerization of Acrylonitrile and Comonomer Mixtures. Starch/Starke 1978, 30, 237–242. [Google Scholar] [CrossRef]
- Athawale, V.D.; Lele, V. Thermal studies on granular maize starch and its graft copolymers with vinyl monomers. Starch/Starke 2000, 52, 205–213. [Google Scholar] [CrossRef]
- Trivedi, J.H.; Prajapati, M.K. Carboxymethyl Sodium Alginate: Synthesis and Characterization. J. Pure Appl. Sci.-Prajna 2015, 22–23, 24–28. [Google Scholar]
- Brockway, C.E.; Seaberg, P.A. Grafting of polyacrylonitrile to granular corn starch. J. Polym. Sci. Part A Polym. Chem. 1967, 5, 1313–1326. [Google Scholar] [CrossRef]







| Sample | IDT | FDT | T10 | T50 | Temperature (°C) at Weight Loss | Temperature Range | Tmax | Weight Loss | Char Yield at 800 °C | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (°C) | 20% | 40% | 60% | (°C) | (°C) | (°C) | (%) | |
| S1 | 140 | 796.53 | 138.14 | 271.17 | 191 | 219 | 459 | 50–100 | -- | 7.19 | 19.28 |
| 135.95–280.85 | 212.88 (34.52) a | 40.64 | |||||||||
| 552.40–795.81 | 701.51 (71.12) a | 19.35 | |||||||||
| S2 | 140 | 798.72 | 215.99 | 588.03 | 274.46 | 482.04 | 683.05 | 50–100 | -- | 2.09 | 30.88 |
| 66.88–136.13 | 92.82 (2.15) a | 2.20 | |||||||||
| 136.13–239.93 | 211.79 (9.27) a | 9.30 | |||||||||
| 239.93–317.77 | 270.26 (18.95) a | 11.88 | |||||||||
| 341.53–430.15 | 384.83 (31.80) a | 8.47 | |||||||||
| 501.60–776.24 | 592.42 (50.43) a | 25.56 | |||||||||
| S3 | 97.71 | 797.81 | 87.35 | 488.62 | 234.44 | 387.03 | 604.29 | 50–100 | -- | 9.07 | 20.42 |
| 154.41–263.50 | 233.35 (20.26) a | 9.33 | |||||||||
| 263.50–469.99 | 378.25 (38.41) a | 24.21 | |||||||||
| 530.65–782.64 | 611.79 (61.79) a | 25.93 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivedi, J.; Chourasia, A. Sodium Salt of Partially Carboxymethylated Sodium Alginate-g-Poly(acrylonitrile): I. Photo-Induced Synthesis, Characterization, and Alkaline Hydrolysis. Gels 2023, 9, 410. https://doi.org/10.3390/gels9050410
Trivedi J, Chourasia A. Sodium Salt of Partially Carboxymethylated Sodium Alginate-g-Poly(acrylonitrile): I. Photo-Induced Synthesis, Characterization, and Alkaline Hydrolysis. Gels. 2023; 9(5):410. https://doi.org/10.3390/gels9050410
Chicago/Turabian StyleTrivedi, Jignesh, and Arvind Chourasia. 2023. "Sodium Salt of Partially Carboxymethylated Sodium Alginate-g-Poly(acrylonitrile): I. Photo-Induced Synthesis, Characterization, and Alkaline Hydrolysis" Gels 9, no. 5: 410. https://doi.org/10.3390/gels9050410
APA StyleTrivedi, J., & Chourasia, A. (2023). Sodium Salt of Partially Carboxymethylated Sodium Alginate-g-Poly(acrylonitrile): I. Photo-Induced Synthesis, Characterization, and Alkaline Hydrolysis. Gels, 9(5), 410. https://doi.org/10.3390/gels9050410