Functionalized Collagen/Poly(ethylene glycol) Diacrylate Interpenetrating Network Hydrogel Enhances Beta Pancreatic Cell Sustenance
Abstract
:1. Introduction
2. Results and Discussion
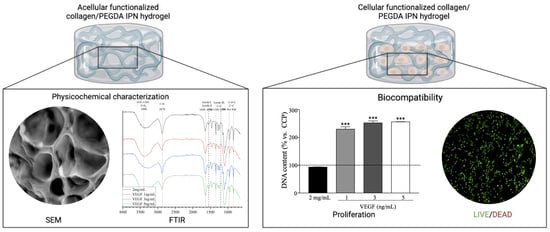
2.1. Physicochemical Characterization
2.1.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.1.2. Scanning Electron Microscopy (SEM)
2.1.3. Rheological Behavior
2.1.4. Swelling and Degradation Rate
2.2. Biocompatibility Assays
2.2.1. Cell Viability and Proliferation
2.2.2. Oxygen Flow
2.2.3. Functional Behavior
3. Conclusions
4. Materials and Methods
4.1. Cell Culture
4.2. Interpenetrating Network (IPN) Hydrogel Fabrication
4.3. Physicochemical Characterization
4.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
4.3.2. Scanning Electron Microscopy (SEM)
4.3.3. Swelling and Degradation Tests
4.3.4. Rheological Analysis
4.3.5. VEGF Release Profile
4.4. Biocompatibility Assays
4.4.1. Cell Viability
4.4.2. Cell Proliferation
4.4.3. Oxygen Uptake
4.4.4. Functional Behavior
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Barnett, R. Type 1 Diabetes. Lancet 2018, 391, 195. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2008, 26, 6. [Google Scholar] [CrossRef]
- Corathers, S.D.; Peavie, S.; Salehi, M. Complications of Diabetes Therapy. Endocrinol. Metab. Clin. N. Am. 2013, 42, 947–970. [Google Scholar] [CrossRef]
- SoRelle, J.A.; Naziruddi, B. Beta Cell Replacement Therapy. In Type 1 Diabetes–Pathogenesis, Genetics and Immunotherapy; Wagner, D., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-362-0. [Google Scholar]
- de Vries, R.; Stell, A.; Mohammed, S.; Hermanns, C.; Martinez, A.H.; Jetten, M.; van Apeldoorn, A. Bioengineering, Biomaterials, and β-Cell Replacement Therapy. In Transplantation, Bioengineering, and Regeneration of the Endocrine Pancreas; Elsevier: Amsterdam, The Netherlands, 2020; pp. 461–486. ISBN 978-0-12-814831-0. [Google Scholar]
- Shapiro, J.; Bruni, A.; Pepper, A.R.; Gala-Lopez, B.; Abualhassan, N.S. Islet Cell Transplantation for the Treatment of Type 1 Diabetes: Recent Advances and Future Challenges. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 2014, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Primavera, R.; Kevadiya, B.D.; Wang, J.; Buchwald, P.; Thakor, A.S. A Collagen Based Cryogel Bioscaffold That Generates Oxygen for Islet Transplantation. Adv. Funct. Mater. 2020, 30, 1902463. [Google Scholar] [CrossRef] [PubMed]
- Llacua, L.A.; Faas, M.M.; de Vos, P. Extracellular Matrix Molecules and Their Potential Contribution to the Function of Transplanted Pancreatic Islets. Diabetologia 2018, 61, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Llacua, L.A.; Hoek, A.; de Haan, B.J.; de Vos, P. Collagen Type VI Interaction Improves Human Islet Survival in Immunoisolating Microcapsules for Treatment of Diabetes. Islets 2018, 10, 60–68. [Google Scholar] [CrossRef]
- Llacua, L.A.; Haan, B.J.; Vos, P. Laminin and Collagen IV Inclusion in Immunoisolating Microcapsules Reduces Cytokine-mediated Cell Death in Human Pancreatic Islets. J. Tissue Eng. Regen. Med. 2018, 12, 460–467. [Google Scholar] [CrossRef]
- Youngblood, R.L.; Sampson, J.P.; Lebioda, K.R.; Shea, L.D. Microporous Scaffolds Support Assembly and Differentiation of Pancreatic Progenitors into β-Cell Clusters. Acta Biomater. 2019, 96, 111–122. [Google Scholar] [CrossRef]
- Sánchez-Cardona, Y.; Echeverri-Cuartas, C.E.; López, M.E.L.; Moreno-Castellanos, N. Chitosan/Gelatin/PVA Scaffolds for Beta Pancreatic Cell Culture. Polymers 2021, 13, 2372. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A Tough Act to Follow: Collagen Hydrogel Modifications to Improve Mechanical and Growth Factor Loading Capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Diaz Quiroz, J.F.; Rodriguez, P.D.; Erndt-Marino, J.D.; Guiza, V.; Balouch, B.; Graf, T.; Reichert, W.M.; Russell, B.; Höök, M.; Hahn, M.S. Collagen-Mimetic Proteins with Tunable Integrin Binding Sites for Vascular Graft Coatings. ACS Biomater. Sci. Eng. 2018, 4, 2934–2942. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.M.; Hayda, K.N.; Anseth, K.S. Cell–Matrix Interactions Improve β-Cell Survival and Insulin Secretion in Three-Dimensional Culture. Tissue Eng. Part A 2008, 14, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Pinto, D.J.; Jimenez-Vergara, A.C.; Gharat, T.P.; Hahn, M.S. Characterization of Sequential Collagen-Poly(Ethylene Glycol) Diacrylate Interpenetrating Networks and Initial Assessment of Their Potential for Vascular Tissue Engineering. Biomaterials 2015, 40, 32–42. [Google Scholar] [CrossRef]
- Jimenez-Vergara, A.C.; Zurita, R.; Jones, A.; Diaz-Rodriguez, P.; Qu, X.; Kusima, K.L.; Hahn, M.S.; Munoz-Pinto, D.J. Refined Assessment of the Impact of Cell Shape on Human Mesenchymal Stem Cell Differentiation in 3D Contexts. Acta Biomater. 2019, 87, 166–176. [Google Scholar] [CrossRef]
- Becerra-Bayona, S.M.; Guiza-Arguello, V.R.; Russell, B.; Höök, M.; Hahn, M.S. Influence of Collagen-based Integrin A1 and A2 Mediated Signaling on Human Mesenchymal Stem Cell Osteogenesis in Three Dimensional Contexts. J. Biomed. Mater. Res. A 2018, 106, 2594–2604. [Google Scholar] [CrossRef] [PubMed]
- Zoratto, N.; Matricardi, P. Semi-IPNs and IPN-Based Hydrogels. In Polymeric Gels; Elsevier: Amsterdam, The Netherlands, 2018; pp. 91–124. ISBN 978-0-08-102179-8. [Google Scholar]
- Goh, M.; Hwang, Y.; Tae, G. Epidermal Growth Factor Loaded Heparin-Based Hydrogel Sheet for Skin Wound Healing. Carbohydr. Polym. 2016, 147, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Han, Y.; Xu, H.; Gao, Y.; Yi, T.; Yao, J.; Dong, L.; Cheng, D.; Chen, Z. VEGF-Conjugated Alginate Hydrogel Prompt Angiogenesis and Improve Pancreatic Islet Engraftment and Function in Type 1 Diabetes. Mater. Sci. Eng. C 2016, 59, 958–964. [Google Scholar] [CrossRef]
- Azarpira, N.; Kaviani, M.; Sarvestani, F.S. Incorporation of VEGF-and BFGF-Loaded Alginate Oxide Particles in Acellular Collagen-Alginate Composite Hydrogel to Promote Angiogenesis. Tissue Cell 2021, 72, 101539. [Google Scholar] [CrossRef] [PubMed]
- Ucar, B.; Yusufogullari, S.; Humpel, C. Collagen Hydrogels Loaded with Fibroblast Growth Factor-2 as a Bridge to Repair Brain Vessels in Organotypic Brain Slices. Exp. Brain Res. 2020, 238, 2521–2529. [Google Scholar] [CrossRef]
- Tsekoura, E.K.; Dick, T.; Pankongadisak, P.; Graf, D.; Boluk, Y.; Uludağ, H. Delivery of Bioactive Gene Particles via Gelatin-Collagen-PEG-Based Electrospun Matrices. Pharmaceuticals 2021, 14, 666. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.; Su, D.; Wu, S.; Zhou, J.; Chen, J. Injectable, Self-Healing and PH Responsive Stem Cell Factor Loaded Collagen Hydrogel as a Dynamic Bioadhesive Dressing for Diabetic Wound Repair. J. Mater. Chem. B 2021, 9, 5887–5897. [Google Scholar] [CrossRef]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen Types Analysis and Differentiation by FTIR Spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR Analysis of Natural and Synthetic Collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Torikai, A.; Shibata, H. Effect of Ultraviolet Radiation on Photodegradation of Collagen. J. Appl. Polym. Sci. 1999, 73, 1259–1265. [Google Scholar] [CrossRef]
- Nashchekina, Y.; Nikonov, P.; Mikhailova, N.; Nashchekin, A. Collagen Scaffolds Treated by Hydrogen Peroxide for Cell Cultivation. Polymers 2021, 13, 4134. [Google Scholar] [CrossRef]
- León-Mancilla, B.H.; Araiza-Téllez, M.A.; Flores-Flores, J.O.; Piña-Barba, M.C. Physico-Chemical Characterization of Collagen Scaffolds for Tissue Engineering. J. Appl. Res. Technol. 2016, 14, 77–85. [Google Scholar] [CrossRef]
- Nashchekina, Y.A.; Starostina, A.A.; Trusova, N.A.; Sirotkina, M.Y.; Lihachev, A.I.; Nashchekin, A.V. Molecular and Fibrillar Structure Collagen Analysis by FTIR Spectroscopy. J. Phys. Conf. Ser. 2020, 1697, 012053. [Google Scholar] [CrossRef]
- Rabotyagova, O.S.; Cebe, P.; Kaplan, D.L. Collagen Structural Hierarchy and Susceptibility to Degradation by Ultraviolet Radiation. Mater. Sci. Eng. C 2008, 28, 1420–1429. [Google Scholar] [CrossRef]
- Magalhães, L.S.S.M.; Andrade, D.B.; Bezerra, R.D.S.; Morais, A.I.S.; Oliveira, F.C.; Rizzo, M.S.; Silva-Filho, E.C.; Lobo, A.O. Nanocomposite Hydrogel Produced from PEGDA and Laponite for Bone Regeneration. J. Funct. Biomater. 2022, 13, 53. [Google Scholar] [CrossRef]
- Rahimi Mamaghani, K.; Morteza Naghib, S.; Zahedi, A.; Mozafari, M. Synthesis and Microstructural Characterization of GelMa/PEGDA Hybrid Hydrogel Containing Graphene Oxide for Biomedical Purposes. Mater. Today Proc. 2018, 5, 15635–15644. [Google Scholar] [CrossRef]
- Punyamoonwongsa, P.; Klayya, S.; Sajomsang, W.; Kunyanee, C.; Aueviriyavit, S. Silk Sericin Semi-Interpenetrating Network Hydrogels Based on PEG-Diacrylate for Wound Healing Treatment. Int. J. Polym. Sci. 2019, 2019, 4740765. [Google Scholar] [CrossRef]
- Li, Y.; Xu, T.; Tu, Z.; Dai, W.; Xue, Y.; Tang, C.; Gao, W.; Mao, C.; Lei, B.; Lin, C. Bioactive Antibacterial Silica-Based Nanocomposites Hydrogel Scaffolds with High Angiogenesis for Promoting Diabetic Wound Healing and Skin Repair. Theranostics 2020, 10, 4929–4943. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.J.; Bae, S.K.; Jung, Y.S.; Kim, J.C.; Kim, J.S.; Park, S.K.; Suh, J.S.; Yi, S.J.; Ahn, S.H.; Lim, J.O. Enhanced Wound Healing Using a 3D Printed VEGF-Mimicking Peptide Incorporated Hydrogel Patch in a Pig Model. Biomed. Mater. 2021, 16, 045013. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, Z.; Wu, D.; Gan, W.; Zhu, S.; Li, W.; Tian, J.; Li, L.; Zhou, C.; Lu, L. Antibacterial Poly (Ethylene Glycol) Diacrylate/Chitosan Hydrogels Enhance Mechanical Adhesiveness and Promote Skin Regeneration. Carbohydr. Polym. 2019, 225, 115110. [Google Scholar] [CrossRef]
- Skrzypek, K.; Groot Nibbelink, M.; Van Lente, J.; Buitinga, M.; Engelse, M.A.; De Koning, E.J.P.; Karperien, M.; Van Apeldoorn, A.; Stamatialis, D. Pancreatic Islet Macroencapsulation Using Microwell Porous Membranes. Sci. Rep. 2017, 7, 9186. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, N.C.; Chatterji, P.R. Structural Characteristics and Swelling Behavior of Poly(Ethylene Glycol) Diacrylate Hydrogels. Macromolecules 1996, 29, 1976–1979. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers, 2nd ed.; Vincentz: Hannover, Germany, 2006. [Google Scholar]
- Moreno-Castellanos, N.; Velásquez-Rincón, M.C.; Rodríguez-Sanabria, A.V.; Cuartas-Gómez, E.; Vargas-Ceballos, O. Encapsulation of Beta-Pancreatic Cells in a Hydrogel Based on Alginate and Graphene Oxide with High Potential Application in the Diabetes Treatment. J. Mater. Res. 2023, 38, 2823–2837. [Google Scholar] [CrossRef]
- Barnes, H.A. A Handbook of Elementary Rheology; University of Wales Institute of Non-Newtonian Fluid Mechanics: Aberystwyth, Wales, 2000; ISBN 978-0-9538032-0-0. [Google Scholar]
- Pablos, J.L.; Jiménez-Holguín, J.; Salcedo, S.S.; Salinas, A.J.; Corrales, T.; Vallet-Regí, M. New Photocrosslinked 3D Foamed Scaffolds Based on GelMA Copolymers: Potential Application in Bone Tissue Engineering. Gels 2023, 9, 403. [Google Scholar] [CrossRef]
- Wang, P.; Berry, D.; Moran, A.; He, F.; Tam, T.; Chen, L.; Chen, S. Controlled Growth Factor Release in 3D-Printed Hydrogels. Adv. Healthc. Mater. 2020, 9, 1900977. [Google Scholar] [CrossRef]
- Yang, X.; Huang, L.; Zhou, L.; Xu, H.; Yi, Z. A Photochromic Copolymer Hydrogel Contact Lens: From Synthesis to Application. Int. J. Polym. Sci. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Furst, E.M.; Kiick, K.L. Manipulation of Hydrogel Assembly and Growth Factor Delivery via the Use of Peptide–Polysaccharide Interactions. J. Control. Release 2006, 114, 130–142. [Google Scholar] [CrossRef]
- Tabata, Y.; Miyao, M.; Ozeki, M.; Ikada, Y. Controlled Release of Vascular Endothelial Growth Factor by Use of Collagen Hydrogels. J. Biomater. Sci. Polym. Ed. 2000, 11, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, A.; Yamamoto, S.; Ozeki, M.; Noguchi, T.; Kanatani, I.; Ogawa, O.; Tabata, Y. Collagenous Matrices as Release Carriers of Exogenous Growth Factors. Biomaterials 2004, 25, 4513–4520. [Google Scholar] [CrossRef]
- Silva, A.K.A.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.-W. Growth Factor Delivery Approaches in Hydrogels. Biomacromolecules 2009, 10, 9–18. [Google Scholar] [CrossRef]
- Colombo, P. Swelling-Controlled Release in Hydrogel Matrices for Oral Route. Adv. Drug Deliv. Rev. 1993, 11, 37–57. [Google Scholar] [CrossRef]
- Lustig, S.R.; Peppas, N.A. Solute Diffusion in Swollen Membranes. IX. Scaling Laws for Solute Diffusion in Gels. J. Appl. Polym. Sci. 1988, 36, 735–747. [Google Scholar] [CrossRef]
- Cosgriff-Hernandez, E.; Hahn, M.S.; Russell, B.; Wilems, T.; Munoz-Pinto, D.; Browning, M.B.; Rivera, J.; Höök, M. Bioactive Hydrogels Based on Designer Collagens. Acta Biomater. 2010, 6, 3969–3977. [Google Scholar] [CrossRef]
- Lin, C.; He, Y.; Xu, K.; Feng, Q.; Li, X.; Zhang, S.; Li, K.; Bai, R.; Jiang, H.; Cai, K. Mesenchymal Stem Cells Resist Mechanical Confinement through the Activation of the Cortex during Cell Division. ACS Biomater. Sci. Eng. 2021, 7, 4602–4613. [Google Scholar] [CrossRef]
- Moreno-Castellanos, N.; Cuartas-Gómez, E.; Vargas-Ceballos, O. Collagen-Mesenchymal Stem Cell Spheroids in Suspension Promote High Adipogenic Capacity. Biomed. Mater. 2023, 18, 045013. [Google Scholar] [CrossRef]
- Rice, A.J.; Cortes, E.; Lachowski, D.; Cheung, B.C.H.; Karim, S.A.; Morton, J.P.; del Río Hernández, A. Matrix Stiffness Induces Epithelial–Mesenchymal Transition and Promotes Chemoresistance in Pancreatic Cancer Cells. Oncogenesis 2017, 6, e352. [Google Scholar] [CrossRef]
- Townsend, S.E.; Gannon, M. Extracellular Matrix–Associated Factors Play Critical Roles in Regulating Pancreatic β-Cell Proliferation and Survival. Endocrinology 2019, 160, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Alessandra, G.; Algerta, M.; Paola, M.; Carsten, S.; Cristina, L.; Paolo, M.; Elisa, M.; Gabriella, T.; Carla, P. Shaping Pancreatic β-Cell Differentiation and Functioning: The Influence of Mechanotransduction. Cells 2020, 9, 413. [Google Scholar] [CrossRef]
- Wright, G.L.; Maroulakou, I.G.; Eldridge, J.; Liby, T.L.; Sridharan, V.; Tsichlis, P.N.; Muise-Helmericks, R.C. VEGF Stimulation of Mitochondrial Biogenesis: Requirement of AKT3 Kinase. FASEB J. 2008, 22, 3264–3275. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wang, Q.; Li, C.; Wang, Y.; Chen, X. VEGF Stimulated the Angiogenesis by Promoting the Mitochondrial Functions. Oncotarget 2017, 8, 77020–77027. [Google Scholar] [CrossRef] [PubMed]
- Cheema, U.; Rong, Z.; Kirresh, O.; MacRobert, A.J.; Vadgama, P.; Brown, R.A. Oxygen Diffusion through Collagen Scaffolds at Defined Densities: Implications for Cell Survival in Tissue Models. J. Tissue Eng. Regen. Med. 2012, 6, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.M.; Anseth, K.S. Hydrogel Encapsulation Environments Functionalized with Extracellular Matrix Interactions Increase Islet Insulin Secretion. Matrix Biol. 2008, 27, 667–673. [Google Scholar] [CrossRef]
- Wang, Z.; Thurmond, D.C. Mechanisms of Biphasic Insulin-Granule Exocytosis–Roles of the Cytoskeleton, Small GTPases and SNARE Proteins. J. Cell Sci. 2009, 122, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yan, S.; Xu, X.; Yu, T.; Guo, Z.; Ma, M.; Zhang, Y.; Gu, Z.; Feng, Y.; Du, C.; et al. Three-Dimensional Cell-Culture Platform Based on Hydrogel with Tunable Microenvironmental Properties to Improve Insulin-Secreting Function of MIN6 Cells. Biomaterials 2021, 270, 120687. [Google Scholar] [CrossRef]
- Sankar, K.S.; Altamentova, S.M.; Rocheleau, J.V. Hypoxia Induction in Cultured Pancreatic Islets Enhances Endothelial Cell Morphology and Survival While Maintaining Beta-Cell Function. PLoS ONE 2019, 14, e0222424. [Google Scholar] [CrossRef]
- Vallejo-Giraldo, C.; Genta, M.; Cauvi, O.; Goding, J.; Green, R. Hydrogels for 3D Neural Tissue Models: Understanding Cell-Material Interactions at a Molecular Level. Front. Bioeng. Biotechnol. 2020, 8, 601704. [Google Scholar] [CrossRef] [PubMed]
- Seyedarabi, A.; Cheng, L.; Zachary, I.; Djordjevic, S. Production of Soluble Human Vascular Endothelial Growth Factor VEGF-A165-Heparin Binding Domain in Escherichia Coli. PLoS ONE 2013, 8, e55690. [Google Scholar] [CrossRef] [PubMed]
- Rehmann, M.S.; Skeens, K.M.; Kharkar, P.M.; Ford, E.M.; Maverakis, E.; Lee, K.H.; Kloxin, A.M. Tuning and Predicting Mesh Size and Protein Release from Step Growth Hydrogels. Biomacromolecules 2017, 18, 3131–3142. [Google Scholar] [CrossRef]
- Keyt, B.A.; Berleau, L.T.; Nguyen, H.V.; Chen, H.; Heinsohn, H.; Vandlen, R.; Ferrara, N. The Carboxyl-Terminal Domain(111–165) of Vascular Endothelial Growth Factor Is Critical for Its Mitogenic Potency. J. Biol. Chem. 1996, 271, 7788–7795. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Lee, S.; Bararpour, L.; Yang, F. Long-Term Controlled Protein Release from Poly(Ethylene Glycol) Hydrogels by Modulating Mesh Size and Degradation: Long-Term Controlled Protein Release from Poly(Ethylene Glycol) Hydrogels. Macromol. Biosci. 2015, 15, 1679–1686. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Castellanos, N.; Cuartas-Gómez, E.; Vargas-Ceballos, O. Functionalized Collagen/Poly(ethylene glycol) Diacrylate Interpenetrating Network Hydrogel Enhances Beta Pancreatic Cell Sustenance. Gels 2023, 9, 496. https://doi.org/10.3390/gels9060496
Moreno-Castellanos N, Cuartas-Gómez E, Vargas-Ceballos O. Functionalized Collagen/Poly(ethylene glycol) Diacrylate Interpenetrating Network Hydrogel Enhances Beta Pancreatic Cell Sustenance. Gels. 2023; 9(6):496. https://doi.org/10.3390/gels9060496
Chicago/Turabian StyleMoreno-Castellanos, Natalia, Elías Cuartas-Gómez, and Oscar Vargas-Ceballos. 2023. "Functionalized Collagen/Poly(ethylene glycol) Diacrylate Interpenetrating Network Hydrogel Enhances Beta Pancreatic Cell Sustenance" Gels 9, no. 6: 496. https://doi.org/10.3390/gels9060496
APA StyleMoreno-Castellanos, N., Cuartas-Gómez, E., & Vargas-Ceballos, O. (2023). Functionalized Collagen/Poly(ethylene glycol) Diacrylate Interpenetrating Network Hydrogel Enhances Beta Pancreatic Cell Sustenance. Gels, 9(6), 496. https://doi.org/10.3390/gels9060496