A Preliminary Study on the Release of Bioactive Compounds from Rice Starch Hydrogels Produced by High-Pressure Processing (HPP)
Abstract
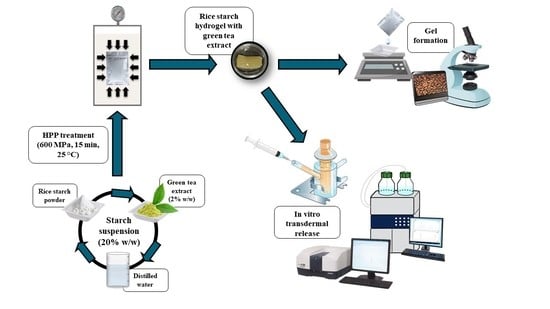
:1. Introduction
2. Results and Discussion
2.1. Gel Formation
2.2. Colour Measurements
2.3. FT-IR Spectra Determination
2.4. In Vitro Transdermal Release by Franz Diffusion Cells
2.4.1. Preliminary Diffusion Study
2.4.2. Release of Bioactive Compounds
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation and Hydrogel Production
4.3. Gel Formation Determination
4.3.1. Microscopy Evaluation
4.3.2. Efficiency Index
4.3.3. Swelling Power
4.4. Colour Measurements
4.5. Fourier Transform Infrared Spectroscopy (FT-IR) Measurements
4.6. Release of Bioactive Compounds
4.6.1. Antioxidant Activity Determination
4.6.2. Total Polyphenol Content Determination
4.6.3. HPLC-PDA Analysis
4.6.4. Release Kinetics Models
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barchielli, B.; Cricenti, C.; Gall, F.; Sabella, E.A.; Liguori, F.; Da Molin, G.; Liguori, G.; Orsi, G.B.; Giannini, A.M.; Ferracuti, S.; et al. Climate changes, natural resources depletion, COVID-19 pandemic, and Russian-Ukrainian war: What is the impact on habits change and mental health? Int. J. Environ. Res. Public Health 2022, 19, 11929. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dhir, A.; Talwar, S.; Chakraborty, D.; Kaur, P. What drives brand love for natural products? The moderating role of household size. J. Retail. Consum. Serv. 2021, 58, 102329. [Google Scholar] [CrossRef]
- Glass, G.E. Cosmeceuticals: The Principles and Practice of Skin Rejuvenation by Nonprescription Topical Therapy. Aesthetic Surg. J. Open Forum 2020, 2, ojaa038. [Google Scholar] [CrossRef]
- Naser, W. Recently emerged bioactive cosmeceuticals for skin rejuvenation: A review. Pharmacologyonline 2020, 2, 243–250. [Google Scholar]
- These, S.; External, A. Cosmeceuticals. The Australasian College of Dermatologists. 2020. Available online: https://www.dermcoll.edu.au/wp-content/uploads/2021/05/Cosmeceuticals-A-Z-of-Skin.pdf (accessed on 20 January 2023).
- Feetham, H.J.; Jeong, H.S.; McKesey, J.; Wickless, H.; Jacobe, H. Skin care and cosmeceuticals: Attitudes and trends among trainees and educators. J. Cosmet. Dermatol. 2018, 17, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Nilforoushzadeh, M.A.; Amirkhani, M.A.; Zarrintaj, P.; Salehi Moghaddam, A.; Mehrabi, T.; Alavi, S.; Mollapour Sisakht, M. Skin care and rejuvenation by cosmeceutical facial mask. J. Cosmet. Dermatol. 2018, 17, 693–702. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-based active substance release systems for cosmetology and dermatology application: A review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef]
- Ismail, H.; Irani, M.; Ahmad, Z. Starch-based hydrogels: Present status and applications. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 411–420. [Google Scholar] [CrossRef]
- Zabot, G.L.; Schaefer Rodrigues, F.; Polano Ody, L.; Vinícius Tres, M.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef]
- Wang, S.; Guo, P. Botanical Sources of Starch; Springer: Singapore, 2020. [Google Scholar]
- Chirani, N.; Yahia, L.H.; Gritsch, L.; Motta, F.L.; Chirani, S.; Farè, S. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4, 1–23. [Google Scholar] [CrossRef]
- Yang, Z.; Chaib, S.; Gu, Q.; Hemar, Y. Impact of pressure on physicochemical properties of starch dispersions. Food Hydrocoll. 2017, 68, 164–177. [Google Scholar] [CrossRef]
- Barba, F.J.; Terefe, N.S.; Buckow, R.; Knorr, D.; Orlien, V. New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods. A review. Food Res. Int. 2015, 77, 725–742. [Google Scholar] [CrossRef]
- Katopo, H.; Song, Y.; Jane, J.L. Effect and mechanism of ultrahigh hydrostatic pressure on the structure and properties of starches. Carbohydr. Polym. 2002, 47, 233–244. [Google Scholar] [CrossRef]
- Błaszczak, W.; Fornal, J.; Kiseleva, V.I.; Yuryev, V.P.; Sergeev, A.I.; Sadowska, J. Effect of high pressure on thermal, structural and osmotic properties of waxy maize and Hylon VII starch blends. Carbohydr. Polym. 2007, 68, 387–396. [Google Scholar] [CrossRef]
- Jiang, B.; Li, W.; Shen, Q.; Hu, X.; Wu, J. Effects of High Hydrostatic Pressure on Rheological Properties of Rice Starch Effects of High Hydrostatic Pressure on Rheological Properties of Rice Starch. Int. J. Food Prop. 2015, 18, 1334–1344. [Google Scholar] [CrossRef]
- Stute, R.; Klingler, R.W.; Boguslawski, S.; Eshtiaghi, M.N.; Knorr, D. Effects of high pressures treatment on starches. Starch/Staerke 1996, 48, 399–408. [Google Scholar] [CrossRef]
- Bauer, B.A.; Knorr, D. The impact of pressure, temperature and treatment time on starches: Pressure-induced starch gelatinisation as pressure time temperature indicator for high hydrostatic pressure processing. J. Food Eng. 2005, 68, 329–334. [Google Scholar] [CrossRef]
- Pei-Ling, L.; Xiao-Song, H.; Qun, S. Effect of high hydrostatic pressure on starches: A review. Starch/Staerke 2010, 62, 615–628. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Sousa, I.; Ferrari, G. Starch-Based Hydrogels Produced by High-Pressure Processing (HPP): Effect of the Starch Source and Processing Time. Food Eng. Rev. 2021, 13, 622–633. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [Green Version]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadon, D.; Wirarti, G.A.; Anwar, E. Novel transdermal ethosomal gel containing green tea (Camellia sinensis L. Kuntze) leaves extract: Formulation and in vitro penetration study. J. Young Pharm. 2017, 9, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Namal Senanayake, S.P.J. Green tea extract: Chemistry, antioxidant properties and food applications—A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Gao, P.; Ogata, Y. CHAMU: An effective approach for improving the recycling of tea waste. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 711, p. 012024. [Google Scholar] [CrossRef]
- Solak, C.N.; Kaleli, A.; Baytut, Ö. The Effect of Green Tea Waste on Growth and Health of Grass Carp (Ctenopharyngodon idellus). Turkish J. Fish. Aquat. Sci. 2016, 16, 953–959. [Google Scholar] [CrossRef]
- Buckow, R.; Heinz, V.; Knorr, D. Effect of high hydrostatic pressure-temperature combinations on the activity of β-glucanase from barley malt. J. Inst. Brew. 2005, 111, 282–289. [Google Scholar] [CrossRef]
- Oh, H.E.; Pinder, D.N.; Hemar, Y.; Anema, S.G.; Wong, M. Effect of high-pressure treatment on various starch-in-water suspensions. Food Hydrocoll. 2008, 22, 150–155. [Google Scholar] [CrossRef]
- Castro, L.M.G.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M. Impact of high pressure on starch properties: A review. Food Hydrocoll. 2020, 106, 105877. [Google Scholar] [CrossRef]
- BeMiller, J.; Whistler, R. Starch: Chemistry and Technology; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Li, W.; Bai, Y.; Mousaa, S.A.S.; Zhang, Q.; Shen, Q. Effect of High Hydrostatic Pressure on Physicochemical and Structural Properties of Rice Starch. Food Bioprocess Technol. 2012, 5, 2233–2241. [Google Scholar] [CrossRef]
- Liu, H.; Lelievre, J.; Ayoung-Chee, W. A study of starch gelatinization using differential scanning calorimetry, X-ray, and birefringence measurements. Carbohydr. Res. 1991, 210, 79–87. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef] [Green Version]
- Tester, R.F.; Morrison, W.R. Swelling and gelatinization of cereal starches. II, Waxy rice starches. Cereal Chem. 1990, 67, 558–563. [Google Scholar]
- Jane, J.; Chen, Y.Y.; Lee, L.F.; McPherson, A.E.; Wong, K.S.; Radosavljevic, M.; Kasemsuwan, T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999, 76, 629–637. [Google Scholar] [CrossRef]
- Kaur, M.; Oberoi, D.P.S.; Sogi, D.S.; Gill, B.S. Physicochemical, morphological and pasting properties of acid treated starches from different botanical sources. J. Food Sci. Technol. 2011, 48, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Alamri, M.; Hussain, S.; Mohamed, A.; Abdo Qasem, A.A.; Ibraheem, M. A study on the effect of black cumin extract on the swelling power, textural, and pasting properties of different starches. Starch/Staerke 2016, 68, 1233–1243. [Google Scholar] [CrossRef]
- Desam, G.P.; Jones, O.G.; Narsimhan, G. Prediction of the effect of sucrose on equilibrium swelling of starch suspensions. J. Food Eng. 2021, 294, 110397. [Google Scholar] [CrossRef]
- Deng, N.; Deng, Z.; Tang, C.; Liu, C.; Luo, S.; Chen, T.; Hu, X. Formation, structure and properties of the starch-polyphenol inclusion complex: A review. Trends Food Sci. Technol. 2021, 112, 667–675. [Google Scholar] [CrossRef]
- Du, J.; Yang, Z.; Xu, X.; Wang, X.; Du, X. Effects of tea polyphenols on the structural and physicochemical properties of high-hydrostatic-pressure-gelatinized rice starch. Food Hydrocoll. 2019, 91, 256–262. [Google Scholar] [CrossRef]
- Smits, A.L.M.; Kruiskamp, P.H.; Van Soest, J.J.G.; Vliegenthart, J.F.G. Interaction between dry starch and plasticisers glycerol or ethylene glycol, measured by differential scanning calorimetry and solid state NMR spectroscopy. Carbohydr. Polym. 2003, 53, 409–416. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Di Nobile, G.; Ferrari, G. Effects of processing conditions and glycerol concentration on rheological and texture properties of starch-based hydrogels produced by high pressure processing (HPP). Int. J. Biol. Macromol. 2020, 159, 590–597. [Google Scholar] [CrossRef]
- Mohamed, R.; Mohd, N.; Nurazzi, N.; Siti Aisyah, M.I.; Mohd Fauzi, F. Swelling and tensile properties of starch glycerol system with various crosslinking agents. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 223, p. 012059. [Google Scholar] [CrossRef] [Green Version]
- Poeloengasih, C.D.; Pranoto, Y.; Hayati, S.N.; Hernawan; Rosyida, V.T.; Prasetyo, D.J.; Jatmiko, T.H.; Apriyana, W.; Suwanto, A. A physicochemical study of sugar palm (Arenga pinnata) starch films plasticized by glycerol and sorbitol. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2016; Volume 1711. [Google Scholar] [CrossRef]
- Ošťádalová, M.; Tremlová, B.; Pokorná, J.; Král, M. Chlorophyll as an indicator of green tea quality. Acta Vet. Brno 2014, 83, S103–S109. [Google Scholar] [CrossRef] [Green Version]
- Kusmita, L.; Puspitaningrum, I.; Limantara, L. Identification, Isolation and Antioxidant Activity of Pheophytin from Green Tea (Camellia sinensis (L.) Kuntze). Procedia Chem. 2015, 14, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Cervera, S.; Salvador, A.; Muguerza, B.; Moulay, L.; Fiszman, S.M. Cocoa fibre and its application as a fat replacer in chocolate muffins. LWT 2011, 44, 729–736. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. Indonesian Journal of Science & Technology How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar]
- van Soest, J.J.G.; De Wit, D.; Tournois, H.; Vliegenthart, J.F.G. FTIR-Gelatinization & Retrogradation of Potato Starch. Starch-Stärke 1994, 46, 453–457. [Google Scholar]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR-ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Stokke, B.T. Polysaccharide hydrogels. Gels 2019, 5, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubens, P.; Snauwaert, J.; Heremans, K.; Stute, R. In situ observation of pressure-induced gelation of starches studied with FTIR in the diamond anvil cell. Carbohydr. Polym. 1999, 39, 231–235. [Google Scholar] [CrossRef]
- Belton, P.S.; Wilson, R.H.; Chenery, D.H. Interaction of group I cations with iota and kappa carrageenans studied by Fourier transform infrared spectroscopy. Int. J. Biol. Macromol. 1986, 8, 247–251. [Google Scholar] [CrossRef]
- Wilson, R.H.; Goodfellow, B.J.; Belton, P.S. Fourier transform infrared spectroscopy for the study of food biopolymers. Top. Catal. 1988, 2, 169–178. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 160. [Google Scholar] [CrossRef] [Green Version]
- Arik Kibar, E.A.; Us, F. Evaluation of structural properties of cellulose ether-corn starch based biodegradable films. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 342–351. [Google Scholar] [CrossRef]
- van Soest, J.J.G.; Tournois, H.; de Wit, D.; Vliegenthart, J.F.G. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr. Res. 1995, 279, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, K.; Aishima, T. Starch gelation process observed by FT-IR/ATR spectrometry with multivariate data analysis. J. Food Sci. 1999, 64, 653–658. [Google Scholar] [CrossRef]
- Larrea-Wachtendorff, D.; Tabilo-Munizaga, G.; Ferrari, G. Potato starch hydrogels produced by high hydrostatic pressure (HHP): A first approach. Polymers 2019, 11, 1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to evaluate skin penetration in vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.C.; De Almeida, P.A.; Polonini, H.C.; Raposo, N.R.B.; De Oliveira Ferreira, A.; Brandão, M.A.F. Green tea in transdermal formulation: Hplc method for quality control and in vitro drug release assays. Quim. Nova 2014, 37, 728–735. [Google Scholar] [CrossRef]
- Baert, B.; Boonen, J.; Burvenich, C.; Roche, N.; Stillaert, F.; Blondeel, P.; Van Bocxlaer, J.; De Spiegeleer, B. A new discriminative criterion for the development of franz diffusion tests for transdermal pharmaceuticals. J. Pharm. Pharm. Sci. 2010, 13, 218–230. [Google Scholar] [CrossRef] [Green Version]
- Batchelder, R.J.; Calder, R.J.; Thomas, C.P.; Heard, C.M. In vitro transdermal delivery of the major catechins and caffeine from extract of Camellia sinensis. Int. J. Pharm. 2004, 283, 45–51. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Mendes de Moraes, F.; Trauthman, S.C.; Zimmer, F.; Pacheco, P.P.; Pont Morisso, F.D.; Ziulkoski, A.L.; Κanis, L.A.; Zepon, K.M. A polysaccharide-based hydrogel as a green platform for enhancing transdermal delivery. Sustain. Chem. Pharm. 2022, 25, 100604. [Google Scholar] [CrossRef]
- Models, T. Transport Models\Zero-Order Model\For zero-order release kinetics. Polymers 2013, 2, 4–7. [Google Scholar]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Darlenski, R.; Surber, C. Glycerol and the skin: Holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Camellia sinensis–Derived Ingredients As Used in Cosmetics. Int. J. Toxicol. 2019, 38, 48S–70S. [Google Scholar] [CrossRef] [PubMed]
- De Maria, S.; Ferrari, G.; Maresca, P.; Pataro, G. Combined effect of high hydrostatic pressure and pulsed light on protein hydrolysis. Chem. Eng. Trans. 2015, 43, 91–96. [Google Scholar] [CrossRef]
- Kusumayanti, H.; Handayani, N.A.; Santosa, H. Swelling Power and Water Solubility of Cassava and Sweet Potatoes Flour. Procedia Environ. Sci. 2015, 23, 164–167. [Google Scholar] [CrossRef] [Green Version]
- Kaur, B.P.; Kaushik, N.; Rao, P.S.; Chauhan, O.P. Effect of High-Pressure Processing on Physical, Biochemical, and Microbiological Characteristics of Black Tiger Shrimp (Penaeus monodon): High-Pressure Processing of Shrimp. Food Bioprocess Technol. 2013, 6, 1390–1400. [Google Scholar] [CrossRef]
- Mauriello, E.; Ferrari, G.; Donsì, F. Effect of formulation on properties, stability, carvacrol release and antimicrobial activity of carvacrol emulsions. Colloids Surf. B Biointerfaces 2021, 197, 111424. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of pulsed electric field in the production of juice and extraction of bioactive compounds from blueberry fruits and their by-products. J. Food Sci. Technol. 2015, 52, 5898–5905. [Google Scholar] [CrossRef]
- Carpentieri, S.; Režek Jambrak, A.; Ferrari, G.; Pataro, G. Pulsed Electric Field-Assisted Extraction of Aroma and Bioactive Compounds From Aromatic Plants and Food By-Products. Front. Nutr. 2022, 8, 1355. [Google Scholar] [CrossRef]




| HPP Treated Samples | Gel Formation Parameters | |
|---|---|---|
| Efficiency Index | Swelling Power (g/gdry starch) | |
| Rice starch hydrogels (control) | ||
| Rice starch hydrogels loaded with green tea extract | ||
| Rice starch hydrogels loaded with green tea extract and glycerol | ||
| Ash (g/100 gDW) | Protein (g/100 gDW) | Fat (g/100 gDW) | Carbohydrate (g/100 gDW) | Total Dietary Fiber (g/100 gDW) |
|---|---|---|---|---|
| 2.1 | 9.7 | 0.5 | 61.9 | 25.3 |
| HPP Hydrogel | Colour Parameters | ||||
|---|---|---|---|---|---|
| L* | a* | b* | WI | ΔEab* | |
| Control hydrogel | 45.6 ± | −0.07 ± | −2.02 ± | - | - |
| Hydrogel with green tea extract | 39.84 ± | −1.21 ± | 22.22 ± | 36.3 ± 0.69 | 24.5 ± 0.48 |
| Receptor Fluid | The Cumulative Amount of Bioactive Released | Inference |
|---|---|---|
| Phosphate buffer solution (PBS), pH 7.4 |
| Foam heap during the time of release |
| PBS/10% of ethanol (1:1) |
| Evaporation issues |
| Citrate-phosphate buffer, pH 5.5 |
| Passage of extract residues |
| Artificial human sweat |
| Homogeneous and transparent solution |
| Sample | Gallic Acid (mg/L) | Catechin (mg/L) | Epicatechin (mg/L) | Rutin (mg/L) |
|---|---|---|---|---|
| 30 min | / | 4.10 | 13.95 | 0.72 |
| 2 h | 1.09 | 6.62 | 32.18 | 1.19 |
| 4 h | 1.21 | 6.17 | 52.80 | 1.28 |
| 8 h | 1.50 | 6.80 | 76.63 | 1.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Aniello, A.; Koshenaj, K.; Ferrari, G. A Preliminary Study on the Release of Bioactive Compounds from Rice Starch Hydrogels Produced by High-Pressure Processing (HPP). Gels 2023, 9, 521. https://doi.org/10.3390/gels9070521
D’Aniello A, Koshenaj K, Ferrari G. A Preliminary Study on the Release of Bioactive Compounds from Rice Starch Hydrogels Produced by High-Pressure Processing (HPP). Gels. 2023; 9(7):521. https://doi.org/10.3390/gels9070521
Chicago/Turabian StyleD’Aniello, Anna, Katerina Koshenaj, and Giovanna Ferrari. 2023. "A Preliminary Study on the Release of Bioactive Compounds from Rice Starch Hydrogels Produced by High-Pressure Processing (HPP)" Gels 9, no. 7: 521. https://doi.org/10.3390/gels9070521
APA StyleD’Aniello, A., Koshenaj, K., & Ferrari, G. (2023). A Preliminary Study on the Release of Bioactive Compounds from Rice Starch Hydrogels Produced by High-Pressure Processing (HPP). Gels, 9(7), 521. https://doi.org/10.3390/gels9070521