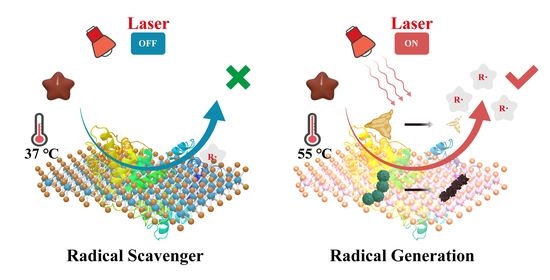
On-Demand Free Radical Release by Laser Irradiation for Photothermal-Thermodynamic Biofilm Inactivation and Tooth Whitening
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of MB NSs
2.2. Evaluating the Stability of AIPH and Its Dye Degradation Capability
2.3. Regulating the Release of Alkyl Free Radicals with MB NSs
2.4. Treatment of Planktonic MRSA and S. mutans In Vitro
2.5. Treatment of S. mutans Biofilm In Vitro
2.6. Preparation of MB NSs-AIPH-Carbomer Composite Hydrogel (MBA-CB Gel)
2.7. Tooth Whitening via Laser-Induced Photothermal-Thermodynamic Treatment
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Characterization
4.3. Preparation of MB NSs
4.4. Preparation of MBA-CB Gel
4.5. Evaluating the Stability of AIPH at Various Temperatures and the Degradation Effect of Released Alkyl Radicals on Dyes
4.6. MB NSs Scavenging Alkyl Radicals at Physiological Temperature
4.7. MB NSs Promote AIPH Decomposition and Radical Release under Laser Irradiation
4.8. Cytotoxicity of MB NSs with AIPH
4.9. Treatment of Planktonic MRSA In Vitro
4.10. Treatment of Planktonic S. mutans In Vitro
4.11. Treatment of S. mutans Biofilm In Vitro
4.12. Tooth Whitening by Using MBA-CB Gel with Laser Irradiation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Oral Disorders Collaborators; Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Watt, R.G.; Daly, B.; Allison, P.; Macpherson, L.M.D.; Venturelli, R.; Listl, S.; Weyant, R.J.; Mathur, M.R.; Guarnizo-Herreno, C.C.; Celeste, R.K.; et al. Ending the neglect of global oral health: Time for radical action. Lancet 2019, 394, 261–272. [Google Scholar] [CrossRef]
- Bawaskar, H.S.; Bawaskar, P.H. Oral diseases: A global public health challenge. Lancet 2020, 395, 185–186. [Google Scholar] [CrossRef] [Green Version]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontology 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, X.; Jia, Y.; Huang, M.; Wang, F.; Zhang, X.; Bai, Y.; Yuan, G.; Wang, Y. Piezo-catalysis for nondestructive tooth whitening. Nat. Commun. 2020, 11, 1328. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, J.S.; Lins-Filho, P.C.; Dias, M.F.; Silva, M.F.; Guimaraes, R.P. Does comsumption of staining drinks compromise the result of tooth whitening? J. Clin. Exp. Dent. 2019, 11, e1012–e1017. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Trombini, J.; Scribante, A. Evaluation of Children Caries Risk Factors: A Narrative Review of Nutritional Aspects, Oral Hygiene Habits, and Bacterial Alterations. Children 2022, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.S.; Koo, H. Targeted, triggered drug delivery to tumor and biofilm microenvironments. Nanomedicine 2016, 11, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Tay, F.R.; Niu, L.N.; Chen, J.H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral Sci. 2019, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Krzysciak, W.; Jurczak, A.; Koscielniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.Y.; Fang, Z.H.; Li, Q.L.; Cao, C.Y. A tooth-binding antimicrobial peptide to prevent the formation of dental biofilm. J. Mater. Sci. Mater. Med. 2019, 30, 1–9. [Google Scholar] [CrossRef]
- Carey, C.M. Tooth Whitening: What We Now Know. J. Evid. Based Dent. Pract. 2014, 14, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Roman-Rodriguez, J.L.; Agustin-Panadero, R.; Roig-Vanaclocha, A.; Amengual, J. A tooth whitening and chemical abrasive protocol for the treatment of developmental enamel defects. J. Prosthet. Dent. 2020, 123, 379–383. [Google Scholar] [CrossRef]
- Soeteman, G.; Valkenburg, C.; Van der Weijden, G.; Van Loveren, C.; Bakker, E.; Slot, D.E. Whitening dentifrice and tooth surface discoloration—A systematic review and meta-analysis. Int. J. Dent. Hyg. 2018, 16, 24–35. [Google Scholar] [CrossRef]
- Markovic, L.; Jordan, R.A.; Lakota, N.; Gaengler, P. Micromorphology of Enamel Surface After Vital Tooth Bleaching. J. Endod. 2007, 33, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Wongpraparatana, I.; Matangkasombut, O.; Thanyasrisung, P.; Panich, M. Effect of vital tooth bleaching on surface roughness and streptococcal biofilm formation on direct tooth-colored restorative materials. Oper. Dent. 2018, 43, 51–59. [Google Scholar] [CrossRef]
- Jenkins, R.R. Free radical chemistry: Relationship to exercise. Sports Med. 1988, 5, 156–170. [Google Scholar] [CrossRef]
- Fan, X.; Yang, F.; Nie, C.; Ma, L.; Cheng, C.; Haag, R. Biocatalytic Nanomaterials: A New Pathway for Bacterial Disinfection. Adv. Mater. 2021, 33, e2100637. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Wan, Y.; Li, X.; Cui, X.; Li, S.; Lee, C.S. Recent Progress of Alkyl Radicals Generation-Based Agents for Biomedical Applications. Adv. Healthc. Mater. 2021, 10, 2100055. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Zhang, W.; Burek, B.O.; Fernández-Fueyo, E.; Alcalde, M.; Bloh, J.Z.; Hollmann, F. Selective activation of C–H bonds in a cascade process combining photochemistry and biocatalysis. Angew. Chem. Int. Ed. 2017, 56, 15451–15455. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Y.; Zhang, R.; Yu, Q.; Liu, Y.; Liu, Y. Glucose-Activated Nanoconfinement Supramolecular Cascade Reaction in Situ for Diabetic Wound Healing. ACS Nano 2022, 16, 9929–9937. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, H.; Li, R.; Wang, Y.; Wang, Z.; Feng, L. Multifunctional Self-Assembly with NIR Light-Activated Cascade Effect for Improving Local Treatment on Solid Tumors. ACS Appl. Mater. Interfaces 2022, 14, 14087–14096. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, L.; Li, J.; Cao, J.; Sun, Y.; Wang, X.; Luo, J.; Zeng, Y.; Li, Q.; Zhang, Y.; et al. Metal-Organic Framework (MOF)-Based Ultrasound-Responsive Dual-Sonosensitizer Nanoplatform for Hypoxic Cancer Therapy. Adv. Healthc. Mater. 2022, 11, e2101946. [Google Scholar] [CrossRef]
- Meng, Z.; Chao, Y.; Zhou, X.; Liang, C.; Liu, J.; Zhang, R.; Cheng, L.; Yang, K.; Pan, W.; Zhu, M.; et al. Near-Infrared-Triggered in Situ Gelation System for Repeatedly Enhanced Photothermal Brachytherapy with a Single Dose. ACS Nano 2018, 12, 9412–9422. [Google Scholar] [CrossRef]
- Pardo, A.; Butera, A.; Giordano, A.; Gallo, S.; Pascadopoli, M.; Scribante, A.; Albanese, M. Photodynamic Therapy in Non-Surgical Treatment of Periodontitis: A Systematic Review and Meta-Analysis. Appl. Sci. 2023, 13, 1086. [Google Scholar] [CrossRef]
- Liao, W.; Xu, C.; Wu, X.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. Photobleachable cinnamoyl dyes for radical visible photoinitiators. Dye. Pigment. 2020, 178, 108350. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X.R.; Wang, W.L.; Qu, X.Y.; Song, X.J.; Zhang, Y.W.; Zhong, L.P.; Yang, D.P.; Dong, X.C.; Zhao, Y.X. Injectable hydrogel for postoperative synergistic photothermal-chemodynamic tumor and anti-infection therapy. Biomaterials 2022, 280, 121289. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, S.; Xu, K.; Liu, T.; Liang, P.; Niu, M.; Fu, C.; Shao, H.; Yu, J.; Ma, T.; et al. Layered MoS2 Hollow Spheres for Highly-Efficient Photothermal Therapy of Rabbit Liver Orthotopic Transplantation Tumors. Small 2016, 12, 2046–2055. [Google Scholar] [CrossRef]
- Ma, K.; Liao, C.; Huang, L.; Liang, R.; Zhao, J.; Zheng, L.; Su, W. Electrospun PCL/MoS2 Nanofiber Membranes Combined with NIR-Triggered Photothermal Therapy to Accelerate Bone Regeneration. Small 2021, 17, 2104747. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Ren, J.; Qu, X. Glutathione Depletion in a Benign Manner by MoS2-Based Nanoflowers for Enhanced Hypoxia-Irrelevant Free-Radical-Based Cancer Therapy. Small 2019, 15, e1904870. [Google Scholar] [CrossRef]
- Yim, D.; Lee, D.E.; So, Y.; Choi, C.; Son, W.; Jang, K.; Yang, C.S.; Kim, J.H. Sustainable Nanosheet Antioxidants for Sepsis Therapy via Scavenging Intracellular Reactive Oxygen and Nitrogen Species. ACS Nano 2020, 14, 10324–10336. [Google Scholar] [CrossRef]
- Ma, T.; Zhai, X.; Huang, Y.; Zhang, M.; Zhao, X.; Du, Y.; Yan, C. A Smart Nanoplatform with Photothermal Antibacterial Capability and Antioxidant Activity for Chronic Wound Healing. Adv. Healthc. Mater. 2021, 10, 2100033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiu, W.; Gan, S.; Shan, J.; Ren, S.; Yuwen, L.; Weng, L.; Teng, Z.; Wang, L. Antibody-Functionalized MoS2 Nanosheets for Targeted Photothermal Therapy of Staphylococcus aureus Focal Infection. Front. Bioeng. Biotechnol. 2019, 7, 218. [Google Scholar] [CrossRef] [Green Version]
- Yuwen, L.; Yu, H.; Yang, X.; Zhou, J.; Zhang, Q.; Zhang, Y.; Luo, Z.; Su, S.; Wang, L. Rapid preparation of single-layer transition metal dichalcogenide nanosheets via ultrasonication enhanced lithium intercalation. Chem. Commun. 2016, 52, 529–532. [Google Scholar] [CrossRef]
- Yuwen, L.; Xu, F.; Xue, B.; Luo, Z.; Zhang, Q.; Bao, B.; Su, S.; Weng, L.; Huang, W.; Wang, L. General synthesis of noble metal (Au, Ag, Pd, Pt) nanocrystal modified MoS2 nanosheets and the enhanced catalytic activity of Pd-MoS2 for methanol oxidation. Nanoscale 2014, 6, 5762–5769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiu, W.; Sun, Y.; Zhu, D.; Zhang, Q.; Yuwen, L.; Weng, L.; Teng, Z.; Wang, L. RGD-QD-MoS2 nanosheets for targeted fluorescent imaging and photothermal therapy of cancer. Nanoscale 2017, 9, 15835–15845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, Y.; Li, Y.; Qi, X.; Yang, J.; Qi, H.; Li, Q.; Ma, Y.; Zhang, Y.; Zhang, X.; et al. A Bifunctional Zwitterion-Modified Porphyrin for Photodynamic Nondestructive Tooth Whitening and Biofilm Eradication. Adv. Funct. Mater. 2021, 31, 2104799. [Google Scholar] [CrossRef]
- Hu, X.; Xie, L.; Xu, Z.; Liu, S.; Tan, X.; Qian, R.; Zhang, R.; Jiang, M.; Xie, W.; Tian, W. Photothermal-Enhanced Fenton-like Catalytic Activity of Oxygen-Deficient Nanotitania for Efficient and Safe Tooth Whitening. ACS Appl. Mater. Interfaces 2021, 13, 35315–35327. [Google Scholar] [CrossRef]
- Wu, J.M.; Chang, W.E.; Chang, Y.T.; Chang, C.K. Piezo-Catalytic Effect on the Enhancement of the Ultra-High Degradation Activity in the Dark by Single- and Few-Layers MoS2 Nanoflowers. Adv. Mater. 2016, 28, 3718–3725. [Google Scholar] [CrossRef]
- Sun, X.; Xu, D.; Dai, P.; Liu, X.; Tan, F.; Guo, Q. Efficient degradation of methyl orange in water via both radical and non-radical pathways using Fe-Co bimetal-doped MCM-41 as peroxymonosulfate activator. Chem. Eng. J. 2020, 402, 125881. [Google Scholar] [CrossRef]
- Park, S.; Garcia-Esparza, A.T.; Abroshan, H.; Abraham, B.; Vinson, J.; Gallo, A.; Nordlund, D.; Park, J.; Kim, T.R.; Vallez, L. Operando Study of Thermal Oxidation of Monolayer MoS2. Adv. Sci. 2021, 8, 2002768. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, X.; Yang, D.; Song, X.; Li, B.; Wang, W.; Dong, X. Ultrasound-Triggered In Situ Gelation to Overcome Tumor Hypoxia for Enhanced Photodynamic and Sustained Chemotherapy. Adv. Ther. 2021, 4, 2100052. [Google Scholar] [CrossRef]
- Li, Y.; Xiu, W.; Yang, K.; Wen, Q.; Yuwen, L.; Luo, Z.; Liu, X.; Yang, D.; Xie, X.; Wang, L. A multifunctional Fenton nanoagent for microenvironment-selective anti-biofilm and anti-inflammatory therapy. Mater. Horiz. 2021, 8, 1264–1271. [Google Scholar] [CrossRef]
- Gu, M.; Jiang, S.; Xu, X.; Wu, M.Y.; Chen, C.; Yuan, Y.; Chen, Q.; Sun, Y.; Chen, L.; Shen, C.; et al. Simultaneous Photodynamic Eradication of Tooth Biofilm and Tooth Whitening with an Aggregation-Induced Emission Luminogen. Adv. Sci. 2022, 9, e2106071. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Kravchuk, O.; Skouroumounis, G.K.; Taylor, D.K. Microwave-assisted and conventional phenolic and colour extraction from grape skins of commercial white and red cultivars at veraison and harvest. J. Clean. Prod. 2020, 275, 122671. [Google Scholar] [CrossRef]
- Su, I.H.; Lee, C.F.; Su, Y.P.; Wang, L.H. Evaluating a Cobalt-Tetraphenylporphyrin Complex, Functionalized with a Reduced Graphene Oxide Nanocomposite, for Improved Tooth Whitening. J. Esthet. Restor. Dent. 2016, 28, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Trapani, B.; Gallo, S.; Radu, M.; Scribante, A. Biomimetic hydroxyapatite paste for molar-incisor hypomineralization: A randomized clinical trial. Oral Dis. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Andrea, B.; Carolina, M.; Gallo, S.; Pascadopoli, M.; Quintini, M.; Lelli, M.; Tarterini, F.; Foltran, I.; Scribante, A. Biomimetic Action of Zinc Hydroxyapatite on Remineralization of Enamel and Dentin: A Review. Biomimetics 2023, 8, 71. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Scardina, G.A.; Pezzullo, S.; Scribante, A. Home Oral Care Domiciliary Protocol for the Management of Dental Erosion in Rugby Players: A Randomized Clinical Trial. J. Clin. Med. 2022, 11, 4893. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Liu, Y.; Ding, M.; Yuwen, L.; Wang, L. On-Demand Free Radical Release by Laser Irradiation for Photothermal-Thermodynamic Biofilm Inactivation and Tooth Whitening. Gels 2023, 9, 554. https://doi.org/10.3390/gels9070554
Zhang Q, Liu Y, Ding M, Yuwen L, Wang L. On-Demand Free Radical Release by Laser Irradiation for Photothermal-Thermodynamic Biofilm Inactivation and Tooth Whitening. Gels. 2023; 9(7):554. https://doi.org/10.3390/gels9070554
Chicago/Turabian StyleZhang, Qi, Yuan Liu, Meng Ding, Lihui Yuwen, and Lianhui Wang. 2023. "On-Demand Free Radical Release by Laser Irradiation for Photothermal-Thermodynamic Biofilm Inactivation and Tooth Whitening" Gels 9, no. 7: 554. https://doi.org/10.3390/gels9070554
APA StyleZhang, Q., Liu, Y., Ding, M., Yuwen, L., & Wang, L. (2023). On-Demand Free Radical Release by Laser Irradiation for Photothermal-Thermodynamic Biofilm Inactivation and Tooth Whitening. Gels, 9(7), 554. https://doi.org/10.3390/gels9070554