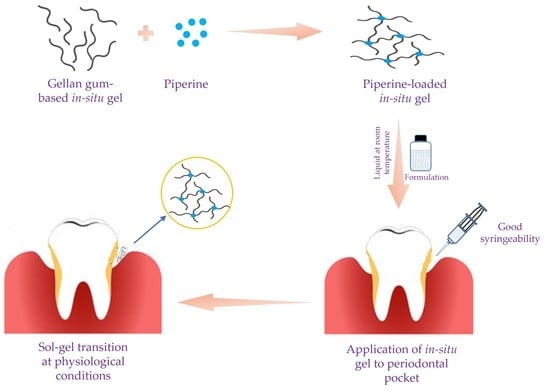
Piperine-Loaded In Situ Gel: Formulation, In Vitro Characterization, and Clinical Evaluation against Periodontitis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pre-Formulation Studies
2.2. In Situ Gel Formulation
2.3. Evaluation of In Situ Gel
2.3.1. Gelation Temperature and Gelation Time
2.3.2. Viscosity Measurement
2.3.3. pH Measurement
2.3.4. Syringeability Study
2.3.5. Drug Content Percentage
2.4. In Vitro Drug Release
2.5. DSC Studies
2.6. Clinical Evaluation of Piperine Gel in Human Patients
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Drug-Excipient Compatibility by Fourier-Transform Infrared (FTIR) Spectroscopy
4.3. In Situ Gel Formulation
4.4. In Vitro Evaluation of Piperine-Loaded In Situ Gel
4.4.1. Gelation Temperature
4.4.2. Gelling Time
4.4.3. Gel pH
4.4.4. Gel Viscosity
4.4.5. Syringeability
4.4.6. Drug Content of the Gel
4.5. Differential Scanning Calorimetric (DSC) Studies
4.6. Drug Release Studies
4.7. Randomized Clinical Trial
4.7.1. Ethical Clearance
4.7.2. Study Design and Study Setting
4.7.3. Study Procedure
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eke, P.I.; Dye, B.A.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef] [Green Version]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The Nexus Between Periodontal Inflammation and Dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Ku, J.K. Guided bone regeneration. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 361–366. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [Green Version]
- D’Elía, N.L.; Silva, R.R.; Sartuqui, J.; Ercoli, D.; Ruso, J.; Messina, P.; Mestres, G. Development and characterization of bilayered periosteum-inspired composite membranes based on sodium alginate-hydroxyapatite nanoparticles. J. Colloid Interface Sci. 2020, 572, 408–420. [Google Scholar] [CrossRef]
- Mota, J.; Yu, N.; Caridade, S.G.; Luz, G.M.; Gomes, M.E.; Reis, R.L.; Jansen, J.A.; Walboomers, X.F.; Mano, J.F. Chitosan/bioactive glass nanoparticle composite membranes for periodontal regeneration. Acta Biomater. 2012, 8, 4173–4180. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Sonoo, P.R.; Siddhartha, R.; Singh, S.K.; Singh, A. Effect of Conventional Periodontal Treatment (Scaling and Root Planing) on Type-2 Diabetic Patient with Moderate Generalized Chronic Periodontitis: A Clinical Study. J. Pharm. Bioallied Sci. 2021, 13, S706–S710. [Google Scholar] [CrossRef]
- Nadig, P.S.; Shah, M.A. Tetracycline as local drug delivery in treatment of chronic periodontitis: A systematic review and meta-analysis. J. Indian Soc. Periodontol. 2016, 20, 576–583. [Google Scholar] [CrossRef]
- Swain, G.P.; Patel, S.; Gandhi, J.; Shah, P. Development of Moxifloxacin Hydrochloride loaded in-situ gel for the treatment of periodontitis: In-vitro drug release study and antibacterial activity. J. Oral Biol. Craniofacial Res. 2019, 9, 190–200. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Hefti, A.F.; Jepsen, S.; Etienne, D.; Walker, C.; Bradshaw, M.H. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis. A review. J. Clin. Periodontol. 2004, 31, 697–707. [Google Scholar] [CrossRef]
- Haque, M.M.; Yerex, K.; Kelekis-Cholakis, A.; Duan, K. Advances in novel therapeutic approaches for periodontal diseases. BMC Oral Health 2022, 22, 492. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.B. The acquisition of antibiotic resistance in the periodontal microflora. Periodontology 2000 1996, 10, 79–88. [Google Scholar] [CrossRef]
- Barca, E.; Cifcibasi, E.; Cintan, S. Adjunctive use of antibiotics in periodontal therapy. J. Istanb. Univ. Fac. Dent. 2015, 49, 55–62. [Google Scholar] [CrossRef]
- Loesche, W.J.; Grossman, N.; Giordano, J. Metronidazole in periodontitis (IV). The effect of patient compliance on treatment parameters. J. Clin. Periodontol. 1993, 20, 96–104. [Google Scholar] [CrossRef]
- Sholapurkar, A.; Sharma, D.; Glass, B.; Miller, C.; Nimmo, A.; Jennings, E. Professionally Delivered Local Antimicrobials in the Treatment of Patients with Periodontitis-A Narrative Review. Dent. J. 2021, 9, 2. [Google Scholar] [CrossRef]
- Lacević, A.; Vranić, E.; Zulić, I. Endodontic-periodontal locally delivered antibiotics. Bosn. J. Basic Med. Sci. 2004, 4, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Rajeshwari, H.R.; Dhamecha, D.; Jagwani, S.; Rao, M.; Jadhav, K.; Shaikh, S.; Puzhankara, L.; Jalalpure, S. Local drug delivery systems in the management of periodontitis: A scientific review. J. Control. Release 2019, 307, 393–409. [Google Scholar] [CrossRef]
- Vyas, S.P.; Sihorkar, V.; Dubey, P.K. Preparation, characterization and in vitro antimicrobial activity of metronidazole bearing lectinized liposomes for intra-periodontal pocket delivery. Pharmazie 2001, 56, 554–560. [Google Scholar] [PubMed]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef] [PubMed]
- Kouchak, M. In situ gelling systems for drug delivery. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e20126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, R.; Kanwar, I.L.; Haider, T.; Pandey, V.; Gour, V.; Soni, V. In situ gel drug delivery system for periodontitis: An insight review. Future J. Pharm. Sci. 2020, 6, 33. [Google Scholar] [CrossRef]
- Boregowda, S.S.; Maggidi, S.R.; Jayaramu, R.A.; Puttegowda, N.; Parbin, N. Development of an In situ Gel Polymer Composite for Local and Sustained Delivery of Drugs in Vaginal Cavity. Drug Deliv. Lett. 2019, 9, 211–221. [Google Scholar] [CrossRef]
- Garala, K.; Joshi, P.; Shah, M.; Ramkishan, A.; Patel, J. Formulation and evaluation of periodontal in situ gel. Int. J. Pharm. Investig. 2013, 3, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Ao, J.; Li, P. A novel in situ gel base of deacetylase gellan gum for sustained ophthalmic drug delivery of ketotifen: In vitro and in vivo evaluation. Drug Des. Dev. Ther. 2015, 9, 3943–3949. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, M.H.; Conway, B.R.; Smith, A.M. Evaluation of gellan gum fluid gels as modified release oral liquids. Int. J. Pharm. 2014, 475, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zheng, Y.; Li, Y.; Zeng, Y.; Kuang, J.; Hou, S.; Li, X. The effect of deacetylated gellan gum on aesculin distribution in the posterior segment of the eye after topical administration. Drug Deliv. 2012, 19, 194–201. [Google Scholar] [CrossRef]
- Galgatte, U.C.; Kumbhar, A.B.; Chaudhari, P.D. Development of in situ gel for nasal delivery: Design, optimization, in vitro and in vivo evaluation. Drug Deliv. 2014, 21, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine-The Bioactive Compound of Black Pepper: From Isolation to Medicinal Formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Piperine and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 173–184. [Google Scholar] [CrossRef]
- Jaisin, Y.; Ratanachamnong, P.; Wongsawatkul, O.; Watthammawut, A.; Malaniyom, K.; Natewong, S. Antioxidant and anti-inflammatory effects of piperine on UV-B-irradiated human HaCaT keratinocyte cells. Life Sci. 2020, 263, 118607. [Google Scholar] [CrossRef]
- Smilkov, K.; Ackova, D.G.; Cvetkovski, A.; Ruskovska, T.; Vidovic, B.; Atalay, M. Piperine: Old Spice and New Nutraceutical? Curr. Pharm. Des. 2019, 25, 1729–1739. [Google Scholar] [CrossRef]
- Zou, L.; Hu, Y.Y.; Chen, W.X. Antibacterial mechanism and activities of black pepper chloroform extract. J. Food Sci. Technol. 2015, 52, 8196–8203. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Huihui, Z.; Li, C. Piperine inhibit inflammation, alveolar bone loss and collagen fibers breakdown in a rat periodontitis model. J. Periodontal Res. 2015, 50, 758–765. [Google Scholar] [CrossRef]
- Aziz, D.M.; Hama, J.R.; Alam, S.M. Synthesising a novel derivatives of piperine from black pepper (Piper nigrum L.). J. Food Meas. Charact. 2015, 9, 324–331. [Google Scholar] [CrossRef]
- Matricardi, P.; Cencetti, C.; Ria, R.; Alhaique, F.; Coviello, T. Preparation and characterization of novel gellan gum hydrogels suitable for modified drug release. Molecules 2009, 14, 3376–3391. [Google Scholar] [CrossRef]
- Liu, L.; Wang, B.; Gao, Y.; Bai, T.-c. Chitosan fibers enhanced gellan gum hydrogels with superior mechanical properties and water-holding capacity. Carbohydr. Polym. 2013, 97, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Kirchmajer, D.M.; Steinhoff, B.; Warren, H.; Clark, R.; in het Panhuis, M. Enhanced gelation properties of purified gellan gum. Carbohydr. Res. 2014, 388, 125–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Gugleva, V.; Michailova, V.; Mihaylova, R.; Momekov, G.; Zaharieva, M.M.; Najdenski, H.; Petrov, P.; Rangelov, S.; Forys, A.; Trzebicka, B.; et al. Formulation and Evaluation of Hybrid Niosomal In Situ Gel for Intravesical Co-Delivery of Curcumin and Gentamicin Sulfate. Pharmaceutics 2022, 14, 747. [Google Scholar] [CrossRef]
- Elmowafy, E.; Cespi, M.; Bonacucina, G.; Soliman, M.E. In situ composite ion-triggered gellan gum gel incorporating amino methacrylate copolymer microparticles: A therapeutic modality for buccal applicability. Pharm. Dev. Technol. 2019, 24, 1258–1271. [Google Scholar] [CrossRef]
- Baloglu, E.; Karavana, S.Y.; Senyigit, Z.A.; Guneri, T. Rheological and mechanical properties of poloxamer mixtures as a mucoadhesive gel base. Pharm. Dev. Technol. 2011, 16, 627–636. [Google Scholar] [CrossRef]
- Rençber, S.; Karavana, S.Y. Formulation and optimization of gellan gum-poloxamer based dexamethasone mucoadhesive in situ gel. J. Res. Pharm. 2020, 201, 529–538. [Google Scholar] [CrossRef]
- Sapra, P.; Patel, D.; Soniwala, M. Development and optimization of in situ periodontal gel containing Levofloxacin for the treatment of periodontal diseases. J. Sci. Innov. Res. 2013, 2, 607–626. [Google Scholar]
- Tiwari, A.; Mahadik, K.R.; Gabhe, S.Y. Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov. 2020, 7, 100027. [Google Scholar] [CrossRef]
- Pentapati, K.C.; Kukkamalla, M.A.; Siddiq, H.; Sabnis, N. Effectiveness of novel herbal dentifrice in control of plaque, gingivitis, and halitosis-Randomized controlled trial. J. Tradit. Complement. Med. 2020, 10, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, C.R.; Kuttan, G. Effect of piperine on the inhibition of nitric oxide (NO) and TNF-alpha production. Immunopharmacol. Immunotoxicol. 2003, 25, 337–346. [Google Scholar] [CrossRef]
- Dwivedi, D.; Singh, V. Effects of the natural compounds embelin and piperine on the biofilm-producing property of Streptococcus mutans. J. Tradit. Complement. Med. 2016, 6, 57–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelkmann, M.; Leichner, C.; Zaichik, S.; Laffleur, F.; Bernkop-Schnürch, A. A gellan gum derivative as in-situ gelling cationic polymer for nasal drug delivery. Int. J. Biol. Macromol. 2020, 158, 1037–1046. [Google Scholar] [CrossRef]
- Grasdalen, H.; Smidsrød, O. Gelation of gellan gum. Carbohydr. Polym. 1987, 7, 371–393. [Google Scholar] [CrossRef]
- Barse, R.; Kokare, C.; Tagalpallewar, A. Influence of hydroxypropylmethylcellulose and poloxamer composite on developed ophthalmic in situ gel: Ex vivo and in vivo characterization. J. Drug Deliv. Sci. Technol. 2016, 33, 66–74. [Google Scholar] [CrossRef]
- Jommanee, N.; Chanthad, C.; Manokruang, K. Preparation of injectable hydrogels from temperature and pH responsive grafted chitosan with tuned gelation temperature suitable for tumor acidic environment. Carbohydr. Polym. 2018, 198, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Sheshala, R.; Quah, S.Y.; Tan, G.C.; Meka, V.S.; Jnanendrappa, N.; Sahu, P.S. Investigation on solution-to-gel characteristic of thermosensitive and mucoadhesive biopolymers for the development of moxifloxacin-loaded sustained release periodontal in situ gels. Drug Deliv. Transl. Res. 2019, 9, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, M.; Surti, Z.; Surti, N.I. Biodegradable in situ Gel for Subcutaneous Administration of Simvastatin for Osteoporosis. Indian J. Pharm. Sci. 2018, 80, 395–399. [Google Scholar] [CrossRef]
- Lee, J.G.; Kim, A.Y.; Kim, D.W.; Kim, Y.J. Determination and risk characterisation of bio-active piperine in black pepper and selected food containing black pepper consumed in Korea. Food Sci. Biotechnol. 2021, 30, 209–215. [Google Scholar] [CrossRef]
- Stasiłowicz, A.; Rosiak, N.; Tykarska, E.; Kozak, M.; Jenczyk, J.; Szulc, P.; Kobus-Cisowska, J.; Lewandowska, K.; Płazińska, A.; Płaziński, W.; et al. Combinations of Piperine with Hydroxypropyl-β-Cyclodextrin as a Multifunctional System. Int. J. Mol. Sci. 2021, 22, 4195. [Google Scholar] [CrossRef]
- Prakasam, A.; Elavarasu, S.S.; Natarajan, R.K. Antibiotics in the management of aggressive periodontitis. J. Pharm. Bioallied Sci. 2012, 4, S252–S255. [Google Scholar] [CrossRef]





| Trials | Gellan Gum (% w/v) | STPP (% w/v) | Consistency at 25 ± 2 °C | Consistency at 35 ± 2 °C |
|---|---|---|---|---|
| 1 | 0.5 | 0.2 | Liquid | Gel |
| 2 | 0.5 | 0.4 | Liquid | Gel |
| 3 | 0.5 | 0.6 | Liquid | Liquid |
| 4 | 0.5 | 0.8 | Liquid | Liquid |
| 5 | 0.5 | 1.0 | Liquid | Liquid |
| 6 | 0.75 | 0.2 | Liquid | Gel |
| 7 | 0.75 | 0.4 | Liquid | Gel |
| 8 | 0.75 | 0.6 | Liquid | Viscous solution |
| 9 | 0.75 | 0.8 | Liquid | Viscous solution |
| 10 | 0.75 | 1.0 | Liquid | Liquid |
| 11 | 1.0 | 0.2 | Gel | Gel |
| 12 | 1.0 | 0.4 | Gel | Gel |
| 13 | 1.0 | 0.6 | Gel | Gel |
| 14 | 1.0 | 0.8 | Gel | Gel |
| 15 | 1.0 | 1.0 | Gel | Gel |
| Formula | Gellan Gum | STPP | Poloxamer 407 | Gelation Temperature (°C) | Gelation Time (s) | pH | Syringeability | Drug Content (%) | Viscosity at 25 °C (cps) | Viscosity at 37 °C (cps) |
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 0.5 | 0.2 | 10 | 31.6 ± 0.6 | 113.0 ± 2.1 | 7.2 ± 0.1 | Pass | 62.5 ± 1.7 | 58.7 ± 1.9 | 287.9 ± 21.3 |
| F2 | 0.5 | 0.2 | 12 | 35.3 ± 0.6 | 50.0 ± 1.5 | 7.0 ± 0.3 | Pass | 87.6 ± 2.9 | 58.8 ± 1.4 | 243.0 ± 19.8 |
| F3 | 0.5 | 0.4 | 10 | 34.3 ± 1.2 | 37.0 ± 1.5 | 7.6 ± 0.4 | Pass | 92.2 ± 2.1 | 59.4 ± 2.1 | 290.2 ± 22.6 |
| F4 | 0.5 | 0.4 | 12 | 34.0 ± 1.0 | 35.0 ± 1.0 | 7.8 ± 0.1 | Pass | 82.5 ± 3.6 | 59.3 ± 1.2 | 287.0 ± 17.8 |
| F5 | 0.75 | 0.2 | 10 | 30.6 ± 0.6 | 63.0 ± 1.2 | 7.0 ± 0.1 | Pass | 57.5 ± 1.9 | 59.2 ± 1.6 | 257.0 ± 13.4 |
| F6 | 0.75 | 0.2 | 12 | 35.0 ± 1.0 | 36.0 ± 1.0 | 7.4 ± 0.3 | Pass | 95.3 ± 2.3 | 58.3 ± 1.7 | 264.0 ± 16.8 |
| F7 | 0.75 | 0.4 | 10 | 31.7 ± 0.6 | 69.0 ± 2.5 | 7.5 ± 0.2 | Pass | 95.1 ± 1.8 | 59.6 ± 1.2 | 280.0 ± 19.4 |
| F8 | 0.75 | 0.4 | 12 | 36.0 ± 1.0 | 43.0 ± 0.6 | 7.6 ± 0.2 | Pass | 85.1 ± 3.1 | 59.0 ± 0.9 | 263.3 ± 14.8 |
| Group | Gender | Frequency | Percentage | Age (Mean ± SD) |
|---|---|---|---|---|
| Group 1 | Male | 9 | 60% | 46.07 ± 10.6 |
| Female | 6 | 40% | ||
| Group 2 | Male | 8 | 53.3% | 44.87 ± 7.1 |
| Female | 7 | 46.7% |
| Parameter | Group | Baseline | Follow-Up | p Value ‡ | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Plaque score | Control | 1.89 | 0.31 | 1.55 | 0.28 | 0.0001 |
| Test | 1.67 | 0.49 | 1.15 | 0.23 | ||
| p value † | 0.140 | |||||
| Gingival index | Control | 1.99 | 0.32 | 1.64 | 0.29 | 0.0003 |
| Test | 1.70 | 0.45 | 1.25 | 0.23 | ||
| p value | 0.053 | |||||
| Pocket depth | Control | 5.53 | 0.52 | 4.40 | 0.63 | 0.0002 |
| Test | 5.47 | 0.52 | 3.27 | 0.59 | ||
| p value | 0.483 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gopalakrishna, P.K.; Jayaramu, R.A.; Boregowda, S.S.; Eshwar, S.; Suresh, N.V.; Abu Lila, A.S.; Moin, A.; Alotaibi, H.F.; Obaidullah, A.J.; Khafagy, E.-S. Piperine-Loaded In Situ Gel: Formulation, In Vitro Characterization, and Clinical Evaluation against Periodontitis. Gels 2023, 9, 577. https://doi.org/10.3390/gels9070577
Gopalakrishna PK, Jayaramu RA, Boregowda SS, Eshwar S, Suresh NV, Abu Lila AS, Moin A, Alotaibi HF, Obaidullah AJ, Khafagy E-S. Piperine-Loaded In Situ Gel: Formulation, In Vitro Characterization, and Clinical Evaluation against Periodontitis. Gels. 2023; 9(7):577. https://doi.org/10.3390/gels9070577
Chicago/Turabian StyleGopalakrishna, Poornima K., Rajamma Abburu Jayaramu, Sateesha Shivally Boregowda, Shruthi Eshwar, Nikhil V. Suresh, Amr Selim Abu Lila, Afrasim Moin, Hadil Faris Alotaibi, Ahmad J. Obaidullah, and El-Sayed Khafagy. 2023. "Piperine-Loaded In Situ Gel: Formulation, In Vitro Characterization, and Clinical Evaluation against Periodontitis" Gels 9, no. 7: 577. https://doi.org/10.3390/gels9070577
APA StyleGopalakrishna, P. K., Jayaramu, R. A., Boregowda, S. S., Eshwar, S., Suresh, N. V., Abu Lila, A. S., Moin, A., Alotaibi, H. F., Obaidullah, A. J., & Khafagy, E. -S. (2023). Piperine-Loaded In Situ Gel: Formulation, In Vitro Characterization, and Clinical Evaluation against Periodontitis. Gels, 9(7), 577. https://doi.org/10.3390/gels9070577