Entrapment of Glucose Oxidase and Catalase in Silica–Calcium–Alginate Hydrogel Reduces the Release of Gluconic Acid in Must
Abstract
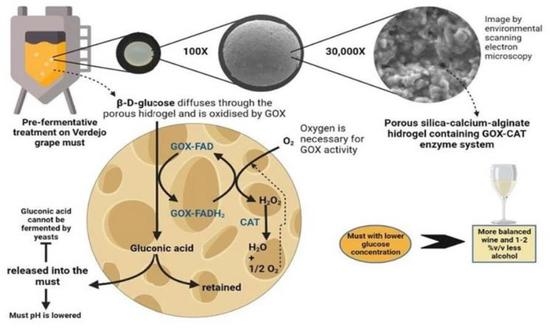
:1. Introduction
2. Results and Discussion
2.1. The Effect of Enzyme Dose, Must pH, and Temperature on the Performance of the Co-Immobilized Enzymes
2.2. Efficiency of the Co-Immobilized and Free GOX
2.3. Operational Stability of the Co-Immobilized GOX
3. Conclusions
- −
- A noteworthy glucose consumption of up to 37.3 g/L was observed with co-immobilized enzymes, resulting in a reduction in the potential alcoholic strength of the must by about 2.0% vol. (v/v).
- −
- A remarkable reduction of up to 73.7% in the estimated gluconic acid concentration was achieved in the co-immobilized enzyme-treated musts, mitigating the risk of an excessive must acidity observed with free enzymes.
- −
- Higher enzyme doses enhanced the pH decrease of must, observing a pH decrease of up to 1.02 with free enzymes and only up to 0.60 with co-immobilized enzymes.
- −
- The rise in the color intensity of the must became less pronounced as the dose of co-immobilized enzymes increased (from 0.39 to 0.33 AU).
- −
- A gradual decline in glucose consumption was observed over eight consecutive cycles of use of the co-immobilized enzymes.
4. Materials and Methods
4.1. Enzymes and Chemical Reagents
4.2. Verdejo Grape Must
4.3. Co-Immobilization of GOX and CAT in Silica–Calcium–Alginate Capsules
4.4. Must Treatment with Co-Immobilized Enzymes
4.5. Efficiency of Co-Immobilized GOX over the Reaction Time
4.6. Operational Stability of Co-Immobilized Enzymes
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, M.N.; Ramos, A.M.; Brands, S. Present and Future Climate Conditions for Winegrowing in Spain. Reg. Environ. Chang. 2016, 16, 617–627. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Kizildeniz, T.; Vucetic, V.; Dai, Z.; Luedeling, E.; van Leeuwen, C.; Gomès, E.; Pascual, I.; Irigoyen, J.J.; Morales, F.; et al. Sensitivity of Grapevine Phenology to Water Availability, Temperature and CO2 Concentration. Front. Environ. Sci. 2016, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.; Tarara, J.M.; Mills, L.J. Spring Temperatures Alter Reproductive Development in Grapevines. Aust. J. Grape Wine Res. 2010, 16, 445–454. [Google Scholar] [CrossRef]
- Aggarwal, S.; Mohite, A.M.; Sharma, N. The Maturity and Tipeness Phenomenon with Regard to the Physiology of Gruits and Begetables: A Review. Bull. Transilv. Univ. Brasov. Ser. II For. Wood Ind. Agric. Food Eng. 2018, 11, 77–88. [Google Scholar]
- Mira, R. Climate Change Associated Effects on Grape and Wine Quality and Production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A. Modified Grape Composition under Climate Change Conditions Requires Adaptations in the Vineyard. OENO One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Pons, A.; Allamy, L.; Schüttler, A.; Rauhut, D.; Thibon, C.; Darriet, P. What Is the Expected Impact of Climate Change on Wine Aroma Compounds and Their Precursors in Grape? OENO One 2017, 51, 141. [Google Scholar] [CrossRef] [Green Version]
- Botezatu, A.; Elizondo, C.; Bajec, M.; Miller, R. Enzymatic Management of pH in White Wines. Molecules 2021, 26, 2730. [Google Scholar] [CrossRef]
- Sam, F.E.; Ma, T.Z.; Salifu, R.; Wang, J.; Jiang, Y.M.; Zhang, B.; Han, S.Y. Techniques for Dealcoholization of Wines: Their Impact on Wine Phenolic Composition, Volatile Composition, and Sensory Characteristics. Foods 2021, 10, 2498. [Google Scholar] [CrossRef]
- Ozturk, B.; Anli, E. Different Techniques for Reducing Alcohol Levels in Wine: A Review. BIO Web Conf. 2014, 3, 02012. [Google Scholar] [CrossRef]
- Schmidtke, L.M.; Blackman, J.W.; Agboola, S.O. Production Technologies for Reduced Alcoholic Wines. J. Food Sci. 2012, 77, R25–R41. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Guindal, A.M.; Tronchoni, J.; Morales, P. Biotechnological Approaches to Lowering the Ethanol Yield during Wine Fermentation. Biomolecules 2021, 11, 1569. [Google Scholar] [CrossRef] [PubMed]
- OIV Resolution OIV-OENO 394B-2012. Correction of the Alcohol Content in Wines. Available online: https://www.oiv.int/public/medias/1432/oiv-oeno-394b-2012-en.pdf (accessed on 22 June 2020).
- Gutiérrez-Gamboa, G.; Zheng, W.; Martínez de Toda, F. Current Viticultural Techniques to Mitigate the Effects of Global Warming on Grape and Wine Quality: A Comprehensive Review. Food Res. Int. 2021, 139, 109946. [Google Scholar] [CrossRef] [PubMed]
- Dubey, M.K.; Zehra, A.; Aamir, M.; Meena, M.; Ahirwal, L.; Singh, S.; Shukla, S.; Upadhyay, R.S.; Bueno-Mari, R.; Bajpai, V.K. Improvement Strategies, Cost Effective Production, and Potential Applications of Fungal Glucose Oxidase (GOD): Current Updates. Front. Microbiol. 2017, 8, 1032. [Google Scholar] [CrossRef] [Green Version]
- Grigoras, A.G. Catalase Immobilization—A Review. Biochem. Eng. J. 2017, 117, 1–20. [Google Scholar] [CrossRef]
- Röcker, J.; Schmitt, M.; Pasch, L.; Ebert, K.; Grossmann, M. The Use of Glucose Oxidase and Catalase for the Enzymatic Reduction of the Potential Ethanol Content in Wine. Food Chem. 2016, 210, 660–670. [Google Scholar] [CrossRef]
- Shen, X.; Yang, M.; Cui, C.; Cao, H. In Situ Immobilization of Glucose Oxidase and Catalase in a Hybrid Interpenetrating Polymer Network by 3D Bioprinting and Its Application. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 568, 411–418. [Google Scholar] [CrossRef]
- Villettaz, J.C. A New Method for the Production of Low Alcohol Wines an Better Balanced Wines. In Proceedings of the 6th Australian Wine Industry Technical Conference, Adelaide, Australia, 14–17 July 1986; Lee, A.T.H., Ed.; Australian Industrial Publishers: Adelaide, Australia, 1986; pp. 125–128. [Google Scholar]
- Heresztyn, T. Conversion of Glucose to Gluconic Acid by Glucose Oxidase Enzyme in Muscat Gordo Juice. Aust. Grapegrow. Winemak. 1987, 4, 25–27. [Google Scholar]
- Pickering, G.J.; Heatherbell, D.A.; Barnes, M.F. Optimising Glucose Conversion in the Production of Reduced Alcohol Wine Using Glucose Oxidase. Food Res. Int. 1998, 31, 685–692. [Google Scholar] [CrossRef]
- Pickering, G.J. Low- and Reduced-Alcohol Wine: A Review. J. Wine Res. 2000, 11, 129–144. [Google Scholar] [CrossRef]
- Pickering, G.J.; Heatherbell, D.A.; Barnes, M.F. GC-MS Analysis of Reduced-Alcohol Müller-Thurgau Wine Produced Using Glucose Oxidase-Treated Luice. LWT Food Sci. Technol. 2001, 34, 89–94. [Google Scholar] [CrossRef]
- Pickering, G.J.; Heatherbell, D.A.; Barnes, M.F. The Production of Reduced- Alcohol Wine Using Glucose Oxidase-Treated Juice. Part I. Composition. Am. Soc. Enol. Vitic. 1999, 50, 291–298. [Google Scholar] [CrossRef]
- Pickering, G.J.; Heatherbell, D.A.; Barnes, M.F. The Production of Reduced-Alcohol Wine Using Glucose Oxidase-Treated Juice. Part II. Stability and SO2-Binding. Am. J. Enol. Vitic. 1999, 50, 299–306. [Google Scholar] [CrossRef]
- Pickering, G.J.; Heatherbell, D.A.; Barnes, M.F. The Production of Reduced-Alcohol Wine Using Glucose Oxidase-Treated Juice. Part III. Sensory. Am. J. Enol. Vitic. 1999, 50, 307–316. [Google Scholar] [CrossRef]
- Biyela, B.N.E.; du Toit, W.J.; Divol, B.; Malherbe, D.F.; van Rensburg, P. The Production of Reduced-Alcohol Wines Using Gluzyme Mono® 10.000 BG-Treated Grape Juice. S. Afr. J. Enol. Vitic. 2009, 30, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Valencia, P.; Espinoza, K.; Ramirez, C.; Franco, W.; Urtubia, A. Technical Feasibility of Glucose Oxidase as a Prefermentation Treatment for Lowering the Alcoholic Degree of Red Wine. Am. J. Enol. Vitic. 2017, 68, 386–389. [Google Scholar] [CrossRef]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose Oxidase—An Overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of Enzyme Activity, Stability and Selectivity via Immobilization Techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Del-Bosque, D.; Vila-Crespo, J.; Ruipérez, V.; Fernández-Fernández, E.; Rodriguez-Nogales, J.M. Silica-Calcium-Alginate Hydrogels for the Co-Immobilization of Glucose Oxidase and Catalase to Reduce the Glucose in Grape Must. Gels 2023, 9, 320. [Google Scholar] [CrossRef]
- Yushkova, E.D.; Nazarova, E.A.; Matyuhina, A.V.; Noskova, A.O.; Shavronskaya, D.O.; Vinogradov, V.V.; Skvortsova, N.N.; Krivoshapkina, E.F. Application of Immobilized Enzymes in Food Industry. J. Agric. Food Chem. 2019, 67, 11553–11567. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An Overview of Technologies for Immobilization of Enzymes and Surface Analysis Techniques for Immobilized Enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Christoph, S.; Fernandes, F.M.; Coradin, T. Immobilization of Proteins in Biopolymer-Silica Hybrid Materials: Functional Properties and Applications. Curr. Org. Chem. 2015, 19, 1669–1676. [Google Scholar] [CrossRef]
- Sperling, L.H.; Hu, R. Interpenetrating Polymer Networks. In Polymer Blends Handbook; Utracki, L., Wilkie, C., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 677–724. [Google Scholar] [CrossRef]
- Simó, G.; Fernández-Fernández, E.; Vila-Crespo, J.; Ruipérez, V.; Rodríguez-Nogales, J.M. Silica-Alginate-Encapsulated Bacteria to Enhance Malolactic Fermentation Performance in a Stressful Environment. Aust. J. Grape Wine Res. 2017, 23, 342–349. [Google Scholar] [CrossRef]
- Simó, G.; Vila-Crespo, J.; Fernández-Fernández, E.; Ruipérez, V.; Rodríguez-Nogales, J.M. Highly Efficient Malolactic Fermentation of Red Wine Using Encapsulated Bacteria in a Robust Biocomposite of Silica-Alginate. J. Agric. Food Chem. 2017, 65, 5188–5197. [Google Scholar] [CrossRef] [PubMed]
- Simó, G.; Fernández-Fernández, E.; Vila-Crespo, J.; Ruipérez, V.; Rodríguez-Nogales, J.M. Research Progress in Coating Techniques of Alginate Gel Polymer for Cell Encapsulation. Carbohydr. Polym. 2017, 170, 1–14. [Google Scholar] [CrossRef]
- Cañete-Rodríguez, A.M.; Santos-Dueñas, I.M.; Jiménez-Hornero, J.E.; Ehrenreich, A.; Liebl, W.; García-García, I. Gluconic Acid: Properties, Production Methods and Applications—An Excellent Opportunity for Agro-Industrial by-Products and Waste Bio-Valorization. Process Biochem. 2016, 51, 1891–1903. [Google Scholar] [CrossRef]
- Kornecki, J.F.; Carballares, D.; Tardioli, P.W.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme Production of D-Gluconic Acid and Glucose Oxidase: Successful Tales of Cascade Reactions. Catal. Sci. Technol. 2020, 10, 5740–5771. [Google Scholar] [CrossRef]
- Ruiz, E.; Busto, M.D.; Ramos-Gómez, S.; Palacios, D.; Pilar-Izquierdo, M.C.; Ortega, N. Encapsulation of Glucose Oxidase in Alginate Hollow Beads to Reduce the Fermentable Sugars in Simulated Musts. Food Biosci. 2018, 24, 67–72. [Google Scholar] [CrossRef]
- Singh, O.V.; Kumar, R. Biotechnological Production of Gluconic Acid: Future Implications. Appl. Microbiol. Biotechnol. 2007, 75, 713–722. [Google Scholar] [CrossRef]
- Ma, Y.; Li, B.; Zhang, X.; Wang, C.; Chen, W. Production of Gluconic Acid and Its Derivatives by Microbial Fermentation: Process Improvement Based on Integrated Routes. Front. Bioeng. Biotechnol. 2022, 10, 864787. [Google Scholar] [CrossRef] [PubMed]
- Ben Messaoud, G.; Sánchez-González, L.; Probst, L.; Jeandel, C.; Arab-Tehrany, E.; Desobry, S. Physico-Chemical Properties of Alginate/Shellac Aqueous-Core Capsules: Influence of Membrane Architecture on Riboflavin Release. Carbohydr. Polym. 2016, 144, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, T.; Reichelt, J. Comparison of Key Characteristics of Industrial Enzymes by Type and Source. In Industrial Enzymology; Godfrey, T., West, S., Eds.; Stockton Press: New York, NY, USA, 1996; pp. 437–479. [Google Scholar]
- Li, H.; Guo, A.; Wang, H. Mechanisms of Oxidative Browning of Wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- El Hosry, L.; Auezova, L.; Sakr, A.; Hajj-Moussa, E. Browning Susceptibility of White Wine and Antioxidant Effect of Glutathione. Int. J. Food Sci. Technol. 2009, 44, 2459–2463. [Google Scholar] [CrossRef]
- Massalha, N.; Basheer, S.; Sabbah, I. Effect of Adsorption and Bead Size of Immobilized Biomass on the Rate of Biodegradation of Phenol at High Concentration Levels. Ind. Eng. Chem. Res. 2007, 46, 6820–6824. [Google Scholar] [CrossRef]
- Simó, G.; Fernández-Fernández, E.; Vila-Crespo, J.; Ruipérez, V.; Rodríguez-Nogales, J.M. Effect of Stressful Malolactic Fermentation Conditions on the Operational and Chemical Stability of Silica-Alginate Encapsulated Oenococcus Oeni. Food Chem. 2019, 276, 643–651. [Google Scholar] [CrossRef]
- Mangas, R.; González, M.R.; Martín, P.; Rodríguez-Nogales, J.M. Impact of Glucose Oxidase Treatment in High Sugar and pH Musts on Volatile Composition of White Wines. LWT 2023, 184, 114975. [Google Scholar] [CrossRef]
- Liese, A.; Hilterhaus, L. Evaluation of Immobilized Enzymes for Industrial Applications. Chem. Soc. Rev. 2013, 42, 6236. [Google Scholar] [CrossRef]
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, Techniques, and Applications of Biocatalyst Immobilization for Industrial Application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme Immobilization: The Quest for Optimum Performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Cui, C.; Fang, Y.; Chen, B.; Tan, T. Glucose Oxidation Performance Is Improved by the Use of a Supramolecular Self-Assembly of Glucose Oxidase and Catalase. Catal. Sci. Technol. 2019, 9, 477–482. [Google Scholar] [CrossRef]
- Milivojevic, M.; Pajić-Lijaković, I.; Levic, S.; Nedovic, V.; Bugarski, B. Alginic Acid: Sources, Modifications and Main Applications. In Alginic Acid: Chemical Structure, Uses and Health Benefits; Moore, A., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2015; pp. 45–88. ISBN 978-1-63463-224-9. [Google Scholar]
- OIV. Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine: Paris, France, 2020; ISBN 978-2-85038-016-7. [Google Scholar]
- Montgomery, D.C. Diseño y Análisis de Experimentos; Editorial Limusa S.A. De C.V.: Mexico City, Mexico, 2004; ISBN 968-18-6156-6. [Google Scholar]








 ), the second 24 h (
), the second 24 h ( ), and the total accumulated consumption in the 48 h (
), and the total accumulated consumption in the 48 h ( ) of pre-fermentative treatment. Bars represent the 95% confidence interval.
) of pre-fermentative treatment. Bars represent the 95% confidence interval.
 ), the second 24 h (
), the second 24 h ( ), and the total accumulated consumption in the 48 h (
), and the total accumulated consumption in the 48 h ( ) of pre-fermentative treatment. Bars represent the 95% confidence interval.
) of pre-fermentative treatment. Bars represent the 95% confidence interval.
 ) and 4.0 (
) and 4.0 ( ) is plotted as a function of cycles of 48 h each (A). Experimental (
) is plotted as a function of cycles of 48 h each (A). Experimental ( ) and estimated (
) and estimated ( ) gluconic acid concentration (g/L) at an initial must pH of 3.8 and experimental (
) gluconic acid concentration (g/L) at an initial must pH of 3.8 and experimental ( ) and estimated (
) and estimated ( ) gluconic acid concentration (g/L) at an initial must pH of 4.0 are plotted as a function of enzyme treatment cycles of 48 h each (B). The pH decrease produced in the must at initial pH levels of 3.8 (
) gluconic acid concentration (g/L) at an initial must pH of 4.0 are plotted as a function of enzyme treatment cycles of 48 h each (B). The pH decrease produced in the must at initial pH levels of 3.8 ( ) and 4.0 (
) and 4.0 ( ) is shown as a function of the enzyme treatment cycles (C). Bars, in all graphs, represent the 95% confidence interval.
) is shown as a function of the enzyme treatment cycles (C). Bars, in all graphs, represent the 95% confidence interval.
 ) and 4.0 (
) and 4.0 ( ) is plotted as a function of cycles of 48 h each (A). Experimental (
) is plotted as a function of cycles of 48 h each (A). Experimental ( ) and estimated (
) and estimated ( ) gluconic acid concentration (g/L) at an initial must pH of 3.8 and experimental (
) gluconic acid concentration (g/L) at an initial must pH of 3.8 and experimental ( ) and estimated (
) and estimated ( ) gluconic acid concentration (g/L) at an initial must pH of 4.0 are plotted as a function of enzyme treatment cycles of 48 h each (B). The pH decrease produced in the must at initial pH levels of 3.8 (
) gluconic acid concentration (g/L) at an initial must pH of 4.0 are plotted as a function of enzyme treatment cycles of 48 h each (B). The pH decrease produced in the must at initial pH levels of 3.8 ( ) and 4.0 (
) and 4.0 ( ) is shown as a function of the enzyme treatment cycles (C). Bars, in all graphs, represent the 95% confidence interval.
) is shown as a function of the enzyme treatment cycles (C). Bars, in all graphs, represent the 95% confidence interval.

| Experimental Factors | Response Variables 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | Enzyme Dose (U/mL) | Initial Must pH | Temperature (°C) | Glucose Consumption (g/L) | Gluconic Acid Concentration (g/L) | pH Decrease | Color Intensity Increase (AU) | Product–Substrate Yield (%) |
| 1 | 1.8 | 4.0 | 20.0 | 30.79 ± 1.72 | 10.36 ± 0.39 | 0.55 ± 0.01 | 0.406 ± 0.001 | 33.64 ± 3.08 |
| 2 | 1.2 | 4.0 | 15.0 | 13.17 ± 1.71 | 8.10 ± 0.39 | 0.51 ± 0.01 | 0.418 ± 0.001 | 61.50 ± 10.85 |
| 3 | 2.4 | 3.8 | 20.0 | 30.56 ± 1.72 | 10.28 ± 0.39 | 0.49 ± 0.01 | 0.340 ± 0.001 | 33.63 ± 3.10 |
| 4 | 1.8 | 3.8 | 15.0 | 26.42 ± 1.72 | 9.60 ± 0.39 | 0.47 ± 0.01 | 0.363 ± 0.001 | 36.32 ± 3.76 |
| 5 | 1.8 | 3.6 | 20.0 | 18.08 ± 1.72 | 7.92 ± 0.39 | 0.33 ± 0.01 | 0.335 ± 0.001 | 43.82 ± 6.24 |
| 6 | 1.2 | 3.8 | 10.0 | 15.50 ± 1.71 | 7.87 ± 0.39 | 0.37 ± 0.01 | 0.423 ± 0.001 | 50.76 ± 8.03 |
| 7 | 1.8 | 3.6 | 10.0 | 23.70 ± 1.72 | 9.74 ± 0.39 | 0.34 ± 0.01 | 0.332 ± 0.001 | 41.08 ± 4.54 |
| 8 | 1.8 | 3.8 | 15.0 | 26.12 ± 1.72 | 9.55 ± 0.39 | 0.43 ± 0.01 | 0.367 ± 0.001 | 36.55 ± 3.82 |
| 9 | 1.8 | 3.8 | 15.0 | 24.83 ± 1.72 | 9.61 ± 0.39 | 0.44 ± 0.01 | 0.365 ± 0.001 | 38.69 ± 4.16 |
| 10 | 2.4 | 3.8 | 10.0 | 30.42 ± 1.72 | 10.06 ± 0.39 | 0.48 ± 0.01 | 0.355 ± 0.001 | 33.06 ± 3.08 |
| 11 | 1.8 | 3.8 | 15.0 | 26.48 ± 1.72 | 9.35 ± 0.39 | 0.43 ± 0.01 | 0.360 ± 0.001 | 35.31 ± 3.69 |
| 12 | 1.2 | 3.6 | 15.0 | 12.24 ± 1.72 | 6.54 ± 0.40 | 0.28 ± 0.01 | 0.361 ± 0.001 | 53.39 ± 10.61 |
| 13 | 2.4 | 3.6 | 15.0 | 35.16 ± 1.72 | 10.07 ± 0.39 | 0.39 ± 0.01 | 0.297 ± 0.001 | 28.64 ± 2.45 |
| 14 | 1.2 | 3.8 | 20.0 | 16.68 ± 1.71 | 7.97 ± 0.39 | 0.32 ± 0.01 | 0.403 ± 0.001 | 47.76 ± 7.16 |
| 15 | 1.8 | 3.8 | 15.0 | 26.22 ± 1.72 | 9.58 ± 0.39 | 0.44 ± 0.01 | 0.365 ± 0.001 | 36.55 ± 3.80 |
| 16 | 1.8 | 4.0 | 10.0 | 24.46 ± 1.72 | 9.93 ± 0.39 | 0.53 ± 0.01 | 0.412 ± 0.001 | 40.58 ± 4.36 |
| 17 | 2.4 | 4.0 | 15.0 | 37.30 ± 1.72 | 11.34 ± 0.39 | 0.60 ± 0.01 | 0.364 ± 0.001 | 30.40 ± 2.39 |
| 18 | 1.8 | 3.8 | 15.0 | 25.43 ± 1.72 | 9.41 ± 0.39 | 0.41 ± 0.01 | 0.362 ± 0.001 | 37.00 ± 3.95 |
| 19 | 1.8 | 3.8 | 15.0 | 26.60 ± 1.72 | 9.80 ± 0.39 | 0.45 ± 0.01 | 0.362 ± 0.001 | 36.84 ± 3.77 |
| Experimental Factors | Response Variables 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | Enzyme Dose (U/mL) | Initial Must pH | Temperature (°C) | Glucose Consumption (g/L) | Gluconic Acid Concentration (g/L) | pH Decrease | Color Intensity Increase (AU) | Product–Substrate Yield (%) |
| 1 | 1.8 | 4.0 | 20.0 | 35.18 ± 1.72 | 35.64 ± 0.69 | 0.78 ± 0.01 | 0.262 ± 0.001 | 101.30 ± 6.83 |
| 2 | 1.2 | 4.0 | 15.0 | 33.88 ± 1.72 | 39.88 ± 0.77 | 0.86 ± 0.01 | 0.311 ± 0.001 | 117.69 ± 8.16 |
| 3 | 2.4 | 3.8 | 20.0 | 43.60 ± 1.72 | 41.14 ± 0.80 | 0.77 ± 0.01 | 0.205 ± 0.001 | 94.36 ± 5.48 |
| 4 | 1.8 | 3.8 | 15.0 | 37.42 ± 1.72 | 36.12 ± 0.70 | 0.84 ± 0.01 | 0.219 ± 0.001 | 96.54 ± 6.22 |
| 5 | 1.8 | 3.6 | 20.0 | 34.63 ± 1.72 | 34.01 ± 0.66 | 0.60 ± 0.01 | 0.169 ± 0.001 | 98.20 ± 6.70 |
| 6 | 1.2 | 3.8 | 10.0 | 21.95 ± 1.72 | 25.93 ± 0.52 | 0.66 ± 0.01 | 0.310 ± 0.001 | 118.12 ± 11.46 |
| 7 | 1.8 | 3.6 | 10.0 | 34.73 ± 1.72 | 25.69 ± 0.52 | 0.59 ± 0.01 | 0.232 ± 0.001 | 73.98 ± 5.11 |
| 8 | 1.8 | 3.8 | 15.0 | 33.29 ± 1.72 | 34.91 ± 0.68 | 0.78 ± 0.01 | 0.235 ± 0.001 | 104.85 ± 7.35 |
| 9 | 1.8 | 3.8 | 15.0 | 38.36 ± 1.72 | 34.77 ± 0.67 | 0.79 ± 0.01 | 0.247 ± 0.001 | 90.66 ± 5.74 |
| 10 | 2.4 | 3.8 | 10.0 | 45.75 ± 1.72 | 42.48 ± 0.83 | 0.83 ± 0.01 | 0.236 ± 0.001 | 92.85 ± 5.23 |
| 11 | 1.8 | 3.8 | 15.0 | 38.62 ± 1.72 | 32.45 ± 0.63 | 0.73 ± 0.01 | 0.245 ± 0.001 | 84.02 ± 5.30 |
| 12 | 1.2 | 3.6 | 15.0 | 15.72 ± 1.72 | 16.04 ± 0.41 | 0.59 ± 0.01 | 0.206 ± 0.001 | 102.08 ± 13.63 |
| 13 | 2.4 | 3.6 | 15.0 | 45.67 ± 1.73 | 52.39 ± 1.04 | 0.73 ± 0.01 | 0.170 ± 0.001 | 114.72 ± 6.51 |
| 14 | 1.2 | 3.8 | 20.0 | 32.60 ± 1.72 | 34.55 ± 0.67 | 0.76 ± 0.01 | 0.210 ± 0.001 | 105.98 ± 7.54 |
| 15 | 1.8 | 3.8 | 15.0 | 37.90 ± 1.72 | 33.42 ± 0.65 | 0.73 ± 0.01 | 0.240 ± 0.001 | 88.19 ± 5.63 |
| 16 | 1.8 | 4.0 | 10.0 | 36.31 ± 1.72 | 28.46 ± 0.56 | 0.81 ± 0.01 | 0.328 ± 0.001 | 78.38 ± 5.19 |
| 17 | 2.4 | 4.0 | 15.0 | 45.92 ± 1.73 | 54.11 ± 1.08 | 1.02 ± 0.01 | 0.262 ± 0.001 | 117.83 ± 6.67 |
| 18 | 1.8 | 3.8 | 15.0 | 34.15 ± 1.72 | 32.98 ± 0.64 | 0.75 ± 0.01 | 0.234 ± 0.001 | 96.58 ± 6.65 |
| 19 | 1.8 | 3.8 | 15.0 | 33.91 ± 1.72 | 33.62 ± 0.65 | 0.78 ± 0.01 | 0.236 ± 0.001 | 99.15 ± 6.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del-Bosque, D.; Vila-Crespo, J.; Ruipérez, V.; Fernández-Fernández, E.; Rodríguez-Nogales, J.M. Entrapment of Glucose Oxidase and Catalase in Silica–Calcium–Alginate Hydrogel Reduces the Release of Gluconic Acid in Must. Gels 2023, 9, 622. https://doi.org/10.3390/gels9080622
del-Bosque D, Vila-Crespo J, Ruipérez V, Fernández-Fernández E, Rodríguez-Nogales JM. Entrapment of Glucose Oxidase and Catalase in Silica–Calcium–Alginate Hydrogel Reduces the Release of Gluconic Acid in Must. Gels. 2023; 9(8):622. https://doi.org/10.3390/gels9080622
Chicago/Turabian Styledel-Bosque, David, Josefina Vila-Crespo, Violeta Ruipérez, Encarnación Fernández-Fernández, and José Manuel Rodríguez-Nogales. 2023. "Entrapment of Glucose Oxidase and Catalase in Silica–Calcium–Alginate Hydrogel Reduces the Release of Gluconic Acid in Must" Gels 9, no. 8: 622. https://doi.org/10.3390/gels9080622
APA Styledel-Bosque, D., Vila-Crespo, J., Ruipérez, V., Fernández-Fernández, E., & Rodríguez-Nogales, J. M. (2023). Entrapment of Glucose Oxidase and Catalase in Silica–Calcium–Alginate Hydrogel Reduces the Release of Gluconic Acid in Must. Gels, 9(8), 622. https://doi.org/10.3390/gels9080622