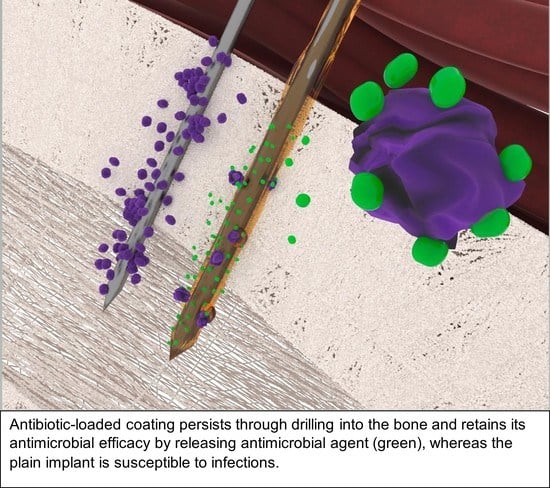
Highly Adhesive Antimicrobial Coatings for External Fixation Devices
Abstract
:1. Introduction
2. Results and Discussion
2.1. PGMA-Based Coatings
2.1.1. Scanning Electron Microscopy
2.1.2. Drug Loading
2.1.3. Film Thickness and Wettability
2.1.4. Drug Release
2.1.5. Activity against Planktonic S. aureus
2.1.6. Zone of Inhibition
2.2. Chitosan Coatings
2.2.1. Scanning Electron Microscopy
2.2.2. Drug Loading
2.2.3. Film Thickness and Wettability
2.2.4. Drug Release
2.2.5. Activity against Planktonic S. aureus
2.2.6. Zone of Inhibition
2.2.7. Anti-Biofilm Activity of the Selected Coatings
2.3. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PGMA-Based Polymers
4.3. Synthesis of Gentamicin Docusate (GD)
4.4. Preparation of Chitosan Solutions
4.5. Preparation of Coating Solutions
4.5.1. PGMA-Based Coating Solutions
4.5.2. Chitosan Coating Solutions
4.5.3. Coating Procedure
4.6. Drilling into the Bone Model
4.7. Scanning Electron Microscopy
4.8. Drug Loading of PGMA-Based Copolymer and Chitosan Gels
4.9. Film Thickness and Wettability
4.10. Sterilization
4.11. Drug Release from PGMA-Based Copolymer and Chitosan Gels
4.12. Antimicrobial Efficacy of PGMA-Based Copolymer and Chitosan Coatings
4.13. Activity against Planktonic Bacteria
4.14. Anti-Biofilm Activity
4.15. Zone of Inhibition
4.16. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Markatos, K.; Tsoucalas, G.; Sgantzos, M. Hallmarks in the history of Orthopaedic Implants for trauma and joint replacement. Acta Med. Hist. Adriat. AMHA 2016, 14, 161–176. [Google Scholar]
- Gollwitzer, H.; Ibrahim, K.; Meyer, H.; Mittelmeier, W.; Busch, R.; Stemberger, A. Antibacterial poly(D,L-lactic acid) coating of medical implants using a biodegradable drug delivery technology. J. Antimicrob. Chemother. 2003, 51, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Fassbender, M.; Minkwitz, S.; Kronbach, Z.; Strobel, C.; Kadow-Romacker, A.; Schmidmaier, G.; Wildemann, B. Local gentamicin application does not interfere with bone healing in a rat model. Bone 2013, 55, 298–304. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Jennison, T.; McNally, M.; Pandit, H. Prevention of infection in external fixator pin sites. Acta Biomater. 2014, 10, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Fragomen, A.T.; Miller, A.O.; Brause, B.D.; Goldman, V.; Rozbruch, S.R. Prophylactic Postoperative Antibiotics May Not Reduce Pin Site Infections After External Fixation. HSS J. 2017, 13, 165–170. [Google Scholar] [PubMed]
- Liu, Y.; Belmont, B.; Wang, Y.; Tai, B.; Holmes, J.; Shih, A. Notched K-wire for low thermal damage bone drilling. Med. Eng. Phys. 2017, 45, 25–33. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, L.; Shih, A.J. Hollow Notched K-Wires for Bone Drilling with through-Tool Cooling. J. Orthop. Res. 2019, 37, 2297–2306. [Google Scholar]
- Luo, Y.; Chen, L.; Finney, F.T.; Park, D.W.; Talusan, P.G.; Holmes, J.R.; Shih, A.J. Evaluation of Heat Generation in Unidirectional Versus Oscillatory Modes During K-Wire Insertion in Bone. J. Orthop. Res. 2019, 37, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Taylor, G.R.; Clarke, N.M.P. A review of K-wire related complications in the emergency management of paediatric upper extremity trauma. Ann. R. Coll. Surg. Engl. 2007, 89, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.R.; Inceoglu, S.; Wongworawat, M.D. Temperature Rise in Kirschner Wires Inserted Using Two Drilling Methods: Forward and Oscillation. Hand 2018, 13, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Batın, S.; Ozan, F.; Gürbüz, K.; Uzun, E.; Kayalı, C.; Altay, T. Migration of a Broken Kirschner Wire after Surgical Treatment of Acromioclavicular Joint Dislocation. Case Rep. Surg. 2016, 2016, 6804670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sananta, P.; Dradjat, R.S.; Julana, R.; Pandiangan, R.A.H.; Sukmajaya, W.P.; Abduh, M. Migration of K-wire into the cavum pleura after the reduction of acromioclavicular dislocation, a case report and review of literature. Int. J. Surg. Case Rep. 2020, 74, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Franssen, B.B.; Schuurman, A.H.; Van der Molen, A.M.; Kon, M. One century of Kirschner wires and Kirschner wire insertion techniques: A historical review. Acta Orthop Belg. 2010, 76, 1–6. [Google Scholar] [PubMed]
- Nowotny, J.; Bischoff, F.; Ahlfeld, T.; Goronzy, J.; Tille, E.; Nimtschke, U.; Biewener, A. Biomechanical comparison of bi- and tricortical k-wire fixation in tension band wiring osteosynthesis. Eur. J. Med. Res. 2019, 24, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durusoy, S.; Öner, K.; Özer, A.; Sevinç, H.F. The effect of the angle between fracture line and Kirschner wires on stability in supracondylar humerus fractures treated with Kirschner wire fixation: A finite element analysis. Jt. Dis. Relat. Surg. 2021, 32, 75–84. [Google Scholar]
- Franssen, B.B.G.M.; van Diest, P.J.; Schuurman, A.H.; Kon, M. Drilling k-wires, what about the osteocytes? An experimental study in rabbits. Arch. Orthop. Trauma Surg. 2008, 128, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Franssen, B.B.G.M.; Schuurman, A.H.; Brouha, P.C.R.; Kon, M. Hammering K-wires is superior to drilling with irrigation. Hand 2009, 4, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Kazmers, N.H.; Fragomen, A.T.; Rozbruch, S.R. Prevention of pin site infection in external fixation: A review of the literature. Strateg. Trauma Limb Reconstr. 2016, 11, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Gil, D.; Shuvaev, S.; Frank-Kamenetskii, A.; Reukov, V.; Gross, C.; Vertegel, A. Novel antibacterial coating on orthopedic wires to eliminate pin tract infections. Antimicrobal Agents Chemother. 2017, 61, 585–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashbaugh, A.G.; Jiang, X.; Zheng, J.; Tsai, A.S.; Kim, W.-S.; Thompson, J.M.; Miller, R.J.; Shahbazian, J.H.; Wang, Y.; Dillen, C.A.; et al. Polymeric nanofiber coating with tunable combinatorial antibiotic delivery prevents biofilm-associated infection in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, E6919–E6928. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Liu, Q.; Zhou, Z.; Lu, W.; Tao, J. Efficacy of tobramycin-loaded coating K-wire in an open-fracture rabbit model contaminated by staphylococcus aureus. Int. J. Clin. Exp. Med. 2017, 10, 6004–6016. [Google Scholar]
- Lucke, M.; Schmidmaier, G.; Sadoni, S.; Wildemann, B.; Schiller, R.; Haas, N.; Raschke, M. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone 2003, 32, 521–531. [Google Scholar] [CrossRef]
- Lucke, M.; Wildemann, B.; Sadoni, S.; Surke, C.; Schiller, R.; Stemberger, A.; Raschke, M.; Haas, N.; Schmidmaier, G. Systemic versus local application of gentamicin in prophylaxis of implant-related osteomyelitis in a rat model. Bone 2005, 36, 770–778. [Google Scholar] [CrossRef]
- Raghavan, R.; Jones, A.; Dwyer, A.J. Should Kirschner wires for fixation of lateral humeral condyle fractures in children be buried or left exposed? A systematic review. Orthop. Traumatol. Surg. Res. 2019, 105, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.F.; Li, Z.J.; Li, C.K.; Bai, S.C.; Li, H. Unburied versus buried wires for fixation of pediatric lateral condyle distal humeral fractures. Medicine 2017, 96, e7736. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, D.G.; Drew, S.J.; Eckersley, R. Kirschner wire pin tract infection rates: A randomized controlled trial between percutaneous and buried wires. J. Hand Surg. 2004, 29, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.; McLaren, A.; Calara, F.; Vernon, B.; McLemore, R. Local Gentamicin Delivery From Resorbable Viscous Hydrogels Is Therapeutically Effective. Clin. Orthop. Relat. Res. 2015, 473, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Vester, H.; Wildemann, B.; Schmidmaier, G.; Stöckle, U.; Lucke, M. Gentamycin delivered from a PDLLA coating of metallic implants: In Vivo and in vitro characterisation for local prophylaxis of implant-related osteomyelitis. Injury 2010, 41, 1053–1059. [Google Scholar] [CrossRef]
- Camathias, C.; Valderrabano, V.; Oberli, H. Routine pin tract care in external fixation is unnecessary: A randomised, prospective, blinded controlled study. Injury 2012, 43, 1969–1973. [Google Scholar] [CrossRef]
- Zhang, B.G.X.; Myers, D.E.; Wallace, G.G.; Brandt, M.; Choong, P.F.M. Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int. J. Mol. Sci. 2014, 15, 11878–11921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Back, D.A.; Bormann, N.; Calafi, A.; Zech, J.; Garbe, L.A.; Müller, M.; Willy, C.; Schmidmaier, G.; Wildemann, B. Testing of antibiotic releasing implant coatings to fight bacteria in combat-associated osteomyelitis—An in-vitro study. Int. Orthop. 2016, 40, 1039–1047. [Google Scholar] [CrossRef]
- Alt, V.; Bitschnau, A.; Österling, J.; Sewing, A.; Meyer, C.; Kraus, R.; Meissner, S.A.; Wenisch, S.; Domann, E.; Schnettler, R. The effects of combined gentamicin-hydroxyapatite coating for cementless joint prostheses on the reduction of infection rates in a rabbit infection prophylaxis model. Biomaterials 2006, 27, 4627–4634. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.D.; Bottagisio, M.M.; Maraldi, S.; Violatto, M.B.; Bortolin, M.; De Vecchi, E.; Bigini, P.; Drago, L.; Romanò, C.L. Vitamin E phosphate coating stimulates bone deposition in implant-related infections in a rat model. Clin. Orthop. Relat. Res. 2018, 476, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.K.; Bjarnsholt, T.; Kragh, K.N.; Aalbæk, B.; Henriksen, N.L.; Blirup, S.A.; Pankoke, K.; Petersen, A.; Jensen, H.E. In Vivo gentamicin susceptibility test for prevention of bacterial biofilms in bone tissue and on implants. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef] [Green Version]
- McMillan, D.J.; Lutton, C.; Rosenzweig, N.; Sriprakash, K.S.; Goss, B.; Stemberger, M.; Schuetz, M.A.; Steck, R. Prevention of Staphylococcus aureus biofilm formation on metallic surgical implants via controlled release of gentamicin. J. Biomed Sci. Eng. 2011, 4, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Yu, X.; Yu, H.; Huang, J.; Zhang, B.; Xiao, J. Development of an anti-infective coating on the surface of intraosseous implants responsive to enzymes and bacteria. J. Nanobiotechnol. 2021, 19, 1–16. [Google Scholar] [CrossRef]
- Stavrakis, A.I.; Zhu, S.; Hegde, V.; Loftin, A.H.; Ashbaugh, A.G.; Niska, J.A.; Miller, L.S.; Segura, T.; Bernthal, N.M. In vivo efficacy of a smart antimicrobial implant coating. J. Bone Jt. Surg. Am. 2016, 98, 1183–1189. [Google Scholar] [CrossRef]
- Kaur, S.; Harjai, K.; Chhibber, S. In Vivo Assessment of Phage and Linezolid Based Implant Coatings for Treatment of Methicillin Resistant S. aureus (MRSA) mediated orthopaedic device related infections. PLoS ONE 2016, 11, e0157626. [Google Scholar] [CrossRef] [Green Version]
- Gulcu, A.; Akman, A.; Demirkan, A.F.; Yorukoglu, A.C.; Kaleli, I.; Bir, F. Fosfomycin addition to poly(D,L-Lactide) Coating does not affect prophylaxis efficacy in rat implant-related infection model, But that of gentamicin does. PLoS ONE 2016, 11, e0165544. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.A.; E Beenken, K.; A Skinner, R.; Meeker, D.G.; Smeltzer, M.S.; O Haggard, W.; Troxel, K.S. Antibiotic-loaded phosphatidylcholine inhibits staphylococcal bone infection. World J. Orthop. 2016, 7, 467–474. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Lucke, M.; Wildemann, B.; Haas, N.P.; Raschke, M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: A review. Injury 2006, 37, S105–S112. [Google Scholar] [CrossRef]
- Borodinov, N.; Gil, D.; Savchak, M.; Gross, C.E.; Yadavalli, N.S.; Ma, R.; Tsukruk, V.V.; Minko, S.; Vertegel, A.; Luzinov, I. En Route to Practicality of the Polymer Grafting Technology: One-Step Interfacial Modification with Amphiphilic Molecular Brushes. ACS Appl. Mater. Interfaces 2018, 10, 13941–13952. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Jung, J. Polymer brush: A promising grafting approach to scaffolds for tissue engineering. BMB Rep. 2016, 49, 655–661. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.S.; Yu, H.; Yeh, P.C.; Yang, J.M.; Hicks, R.F. Atmospheric pressure plasma effects on the adhesive bonding properties of stainless steel and epoxy composites. J. Compos. Mater. 2014, 48, 219–233. [Google Scholar] [CrossRef]
- Tang, S.; Kwon, O.-J.; Lu, N.; Choi, H.-S. Surface Free Energy Changes of Stainless Steel after One Atmospheric Pressure Plasma Treatment. Korean J. Chem. Eng. 2004, 21, 1218–1223. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of chitosan in environmental remediation: A review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef]
- Negi, H.; Verma, P.; Singh, R.K. A comprehensive review on the applications of functionalized chitosan in petroleum industry. Carbohydr. Polym. 2021, 266, 118125. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Eini, M.; Rastegari, A.; Tehrani, M.R. Chitosan as a machine for biomolecule delivery: A review. Carbohydr. Polym. 2021, 256, 117414. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Guan, Y.X.; Yao, S.J.; Zhu, Z.Q. Preparation of ibuprofen-loaded chitosan films for oral mucosal drug delivery using supercritical solution impregnation. Int. J. Pharm. 2014, 473, 434–441. [Google Scholar] [CrossRef]
- Huo, W.; Xie, G.; Zhang, W.; Wang, W.; Shan, J.; Liu, H.; Zhou, X. Preparation of a novel chitosan-microcapsules/starch blend film and the study of its drug-release mechanism. Int. J. Biol. Macromol. 2016, 87, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.W.-Y.; Sun, W.; Bhowmik, A.; Lek, J.Y.; Marinescu, I.; Li, F.; Khun, N.W.; Dong, Z.; Liu, E. Effect of coating thickness on microstructure, mechanical properties and fracture behaviour of cold sprayed Ti6Al4V coatings on Ti6Al4V substrates. Surf. Coat. Technol. 2018, 349, 303–317. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, S.; Wu, H.; Qin, H.; Ren, M.; Lin, J.; Yu, B. Aspirin alleviates orthopedic implant-associated infection. Int. J. Mol. Med. 2019, 44, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Harjai, K.; Chhibber, S. Bacteriophage mediated killing of Staphylococcus aureus in vitro on orthopaedic K wires in presence of linezolid prevents implant colonization. PLoS ONE 2014, 9, e90411. [Google Scholar] [CrossRef] [Green Version]
- Shiels, S.M.; Mangum, L.H.; Wenke, J.C. Revisiting the “race for the surface” in a pre-clinical model of implant infection. Eur. Cell Mater. 2020, 39, 77–95. [Google Scholar] [CrossRef]
- Mehdikhani-Nahrkhalaji, M.; Fathi, M.H.; Mortazavi, V.; Mousavi, S.B.; Hashemi-Beni, B.; Razavi, S.M. Novel nanocomposite coating for dental implant applications in vitro and in vivo evaluation. J. Mater. Sci. Mater. Med. 2012, 23, 485–495. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Stemberger, A.; Haas, N.P.; Raschke, M. Biodegradable Poly(D,L-Lactide) Coating of Implants for Continuous Release of Growth Factors. J. Biomed. Mater. Res. 2001, 58, 449–455. [Google Scholar] [CrossRef]
- Rajan, S.; Srikumar, S.; Tosh, P.; Kumar, L. Effect of lactate versus acetate-based intravenous fluids on acid-base balance in patients undergoing free flap reconstructive surgeries. J. Anesthesiol. Clin. Pharmacol. 2017, 33, 514–519. [Google Scholar]
- Elizondo, E.; Sala, S.; Imbuluzqueta, E.; González, D.; Blanco-Prieto, M.J.; Gamazo, C.; Ventosa, N.; Veciana, J. High loading of gentamicin in bioadhesive PVM/MA nanostructured microparticles using compressed carbon-dioxide. Pharm. Res. 2011, 28, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Gubernator, J.; Drulis-Kawa, Z.; Kozubek, A. A simply and sensitive fluorometric method for determination of gentamicin in liposomal suspensions. Int. J. Pharm. 2006, 327, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Mullins, N.D.; Deadman, B.J.; Moynihan, H.A.; McCarthy, F.O.; Lawrence, S.E.; Thompson, J.; Maguire, A.R. The impact of storage conditions upon gentamicin coated antimicrobial implants. J. Pharm. Anal. 2016, 6, 374–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samara, E.; Moriarty, T.F.; Decosterd, L.A.; Richards, R.G.; Gautier, E.; Wahl, P. Antibiotic stability over six weeks in aqueous solution at body temperature with and without heat treatment that mimics the curing of bone cement. Bone Jt. Res. 2017, 6, 296–306. [Google Scholar] [CrossRef]
- Guillaume, O.; Garric, X.; Lavigne, J.P.; van den Berghe, H.; Coudane, J. Multilayer, degradable coating as a carrier for the sustained release of antibiotics: Preparation and antimicrobial efficacy in vitro. J. Control. Release. 2012, 162, 492–501. [Google Scholar] [CrossRef]













| Controls | PGMA-Based Coating Solutions | Chitosan Coating Solutions |
|---|---|---|
| Gentamicin sulfate (GS) 5% in H2O (plain drug control) | P(GMA25-OEGMA70-LMA5) 10% + GS 5% in H2O | Chitosan Lactate 2.7% + VH 5% in H2O |
| Gentamicin docusate (GD) 5% in MEK (plain drug control) | P(GMA25-LMA75) 10% + GD 5% in MEK | Chitosan Acetate 1.35% + VH 2.5% in H2O |
| Vancomycin Hydrochloride (VH) 5% in H2O (plain drug control) | P(GMA25-OEGMA50-LMA25) 10% + GD 5% in MEK | Chitosan Acetate 1.35% + VH 5% in H2O |
| Polylactic acid (PLA) 10% + GD 5% in MEK (commercial coating control) | P(GMA25-OEGMA70-LMA5) 10% + GD 5% in MEK | Chitosan Acetate 1.35% + VH 10% in H2O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bredikhin, M.; Sawant, S.; Gross, C.; Antonio, E.L.S.; Borodinov, N.; Luzinov, I.; Vertegel, A. Highly Adhesive Antimicrobial Coatings for External Fixation Devices. Gels 2023, 9, 639. https://doi.org/10.3390/gels9080639
Bredikhin M, Sawant S, Gross C, Antonio ELS, Borodinov N, Luzinov I, Vertegel A. Highly Adhesive Antimicrobial Coatings for External Fixation Devices. Gels. 2023; 9(8):639. https://doi.org/10.3390/gels9080639
Chicago/Turabian StyleBredikhin, Mikhail, Sushant Sawant, Christopher Gross, Erik L. S. Antonio, Nikolay Borodinov, Igor Luzinov, and Alexey Vertegel. 2023. "Highly Adhesive Antimicrobial Coatings for External Fixation Devices" Gels 9, no. 8: 639. https://doi.org/10.3390/gels9080639
APA StyleBredikhin, M., Sawant, S., Gross, C., Antonio, E. L. S., Borodinov, N., Luzinov, I., & Vertegel, A. (2023). Highly Adhesive Antimicrobial Coatings for External Fixation Devices. Gels, 9(8), 639. https://doi.org/10.3390/gels9080639