Refining and Validating Thermogravimetric Analysis (TGA) for Robust Characterization and Quality Assurance of Graphene-Related Two-Dimensional Materials (GR2Ms)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
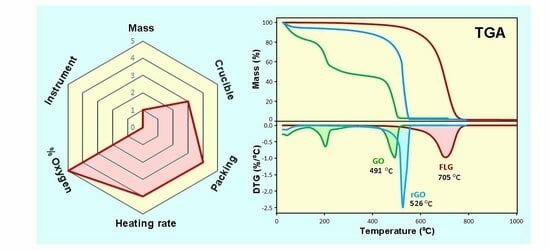
3.1. Optimizing TGA Experimental Conditions for the Characterization of GR2Ms
3.1.1. The Influence of the Mass and Method of Packing the GR2M Sample in the Crucible
- A mass of graphene sample in the range of 2.6–9.3 mg that is freely packed with the same packing density does not cause a significant difference in the TGA results. However, a mass of 5.0 mg with free loading is recommended (applied for a 70 uL crucible) to allow good heat transfer and maintain the consistency of TGA measurements (the exemptions are graphene samples which are ultra-light, fluffy, and highly porous and for which a lower mass ( around 2–3 mg) is sufficient to fully fill a 70 µL crucible using the free-loading method).
- The results show that the packing method has a substantial influence on TGA results, especially when a higher mass is used. It is recommended to freely load (loosely pack) graphene powder in the crucible without pressing to prevent thermal lag (or to allow efficient heat transfer).
3.1.2. The Influence of Open and Closed Crucibles
- An optimal condition that will provide the uniform heat transfer needed for a TGA experiment is the use of an open crucible. However, to avoid the potential loss in case of using very light graphene samples from crucibles during TGA measurements, it is recommended to use a lid with a hole to cover the crucible with a heating rate of 5 °C/min or lower.
- There is an insignificant influence of the type of crucible (alumina vs. platinum) under these conditions, and both types of crucibles can be used with no impact on TGA results.
3.1.3. The Influence of the Heating Rate
- The heating rate has a significant influence on TG/DTG graphs and Tmax values, which are significantly increased by increasing the heating rate.
- The recommended heating rate for TGA of GR2Ms is in the range between 2 °C/min and 10 °C/min and, for consistency, an optimal heating rate of 5 °C/min is recommended. It is not recommended to use a higher heating rate > 20 °C/min that causes a significant widenning of the DTG peaks that might cause hiding to determine the presence of remaining impurities.
3.1.4. The Influence of the Heating Environment (Air/Oxygen and Nitrogen)
- The gas environment during TGA measurements has a significant influence on TG/DTG graphs and their Tmax values, which are significantly decreased by increasing the % of oxygen. Increasing the % of oxygen was found to have a minor influence on the quantitative mass loss determination % of carbon. The gas environment is critical for measurements when Tmax is used as an analytical parameter (a qualitative analysis); therefore, it is important to keep these conditions constant.
- The recommended gas environment for GR2M characterization is air that can provide adequate oxygen for the oxidative decomposition of GR2Ms for both qualitative and quantitative TGAs and is inexpensive.
3.1.5. The Influence of the Sample Gas Flow Rate
- The sample gas flow rate has an influence on TG/DTG graphs and Tmax values that needs to be optimized. This impact is significant, especially at low sample gas flow rates of 0–10 mL/min, which are practically not suitable. Applicable sample gas flow rates for the TGA of GR2Ms are between 25 and 100 mL/min with minor changes in quantitative TGA measurements (mass % C).
- A gas flow rate of 60 mL/min with an air atmosphere is recommended as an optimal condition that will be within the range of most TGA devices and can provide consistent results for both qualitative and quantitative TGAs.
3.1.6. The Influence of TGA Instrument Design (Furnace and Thermobalance)
- These results confirm that there is an insignificant influence of TGA instrument design on TGA results when using the same experimental conditions.
3.2. Validating the Optimized TGA Method for Characterizing GR2Ms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrari, A.C.; Bonaccorso, F.; Fal’Ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.; Palermo, V.; Pugno, N. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Kum, H.; Bae, S.-H.; Shim, J.; Kim, H.; Kong, L.; Meng, Y.; Wang, K.; Kim, C.; Kim, J. Path towards graphene commercialization from lab to market. Nat. Nanotechnol. 2019, 14, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Kauling, A.P.; Seefeldt, A.T.; Pisoni, D.P.; Pradeep, R.C.; Bentini, R.; Oliveira, R.V.B.; Novoselov, K.S.; Castro Neto, A.H. The Worldwide Graphene Flake Production. Adv. Mater. 2018, 30, 1803784. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ji, H.; Cheng, H.-M.; Ruoff, R.S. Mass production and industrial applications of graphene materials. Natl. Sci. 2017, 5, 90–101. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; Grossman, J.C. Water Desalination across Nanoporous Graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Bøggild, P. The war on fake graphene. Nature 2018, 562, 502–503. [Google Scholar] [CrossRef] [PubMed]
- ISO/TS 80004-13:2017; Nanotechnologies—Vocabulary—Part 13: Graphene and Related Two-Dimensional (2D) Materials. ISO: Geneva, Switzerland, 2017.
- ISO/TS 21356-1:2021; Nanotechnologies—Structural Characterization of Graphene. Part 1: Graphene from Powders and Dispersions. ISO: Geneva, Switzerland, 2021; 48p.
- ISO/TR 19733:2019; ISO Nanotechnologies. Matrix of Properties and Measurement Techniques for Graphene and Related Two-Dimensional (2D) Materials. ISO: Geneva, Switzerland, 2019.
- PAS 1201:2018; Properties of Graphene Flakes—Guide. The British Standards Institution (BSI): London, UK, 2018.
- Pollard, A.; Paton, K.; Clifford, C.; Legge, E. Characterisation of the Structure of Graphene. Good Practice Guide No 145; National Physical Laboratory (NPL): Teddington, UK, 2017. [Google Scholar]
- Farivar, F.; Yap, P.L.; Hassan, K.; Tung, T.T.; Tran, D.N.H.; Pollard, A.J.; Losic, D. Unlocking thermogravimetric analysis (TGA) in the fight against “Fake graphene” materials. Carbon 2021, 179, 505–513. [Google Scholar] [CrossRef]
- Farivar, F.; Lay Yap, P.; Karunagaran, R.U.; Losic, D. Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameters. C 2021, 7, 41. [Google Scholar] [CrossRef]
- Shtein, M.; Pri-Bar, I.; Varenik, M.; Regev, O. Characterization of Graphene-Nanoplatelets Structure via Thermogravimetry. Anal. Chem. 2015, 87, 4076–4080. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.L.; Farivar, F.; Jämting, Å.K.; Coleman, V.A.; Gnaniah, S.; Mansfield, E.; Pu, C.; Landi, S.M.; David, M.V.; Flahaut, E.; et al. International Interlaboratory Comparison of Thermogravimetric Analysis of Graphene-Related Two-Dimensional Materials. Anal. Chem. 2023, 95, 5176–5186. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Farivar, F.; Yap, P.L.; Karami, A. Accounting Carbonaceous Counterfeits in Graphene Materials Using the Thermogravimetric Analysis (TGA) Approach. Anal. Chem. 2021, 93, 11859–11867. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.O.; Akinlabi, S.A.; Mashinini, M.P.; Fatoba, S.O.; Ajayi, O.O. Thermo-gravimetric characterization of biomass properties: A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 423, 012175. [Google Scholar] [CrossRef]
- Gabbott, P. Principles and Applications of Thermal Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Brown, M.E. Introduction to Thermal Analysis: Techniques and Applications; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Losic, D.; Farivar, F.; Yap, P.L.; Tung, T.T.; Nine, M.J. New insights on energetic properties of graphene oxide (GO) materials and their safety and environmental risks. Sci. Total Environ. 2022, 848, 157743. [Google Scholar] [CrossRef] [PubMed]








| Evaluated Conditions | Applicable Range | Optimized | Comments |
|---|---|---|---|
| Mass of sample, mg (2–47 mg) | 2–10 | 4–5 mg | Limited by vol. of crucible, powders not fluffy |
| Sample loading in crucible (pressure free or pressure loaded) | Pressure-free | Pressure-free | Tightly packed/pressed sample with higher mass will impact heat transfer and results |
| Type of crucible (Pt or alumina) | Pt or alumina, 60–100 uL | Alumina or Pt | No influence of crucible materials is observed |
| Open or closed crucible (lid with a hole) | Both acceptable. Open can be used for higher heating rating up to 10 °C/min and a lid with ≤5°C/min | Open | For fluffy graphene samples a lid with a hole is a recommended solution with optimized conditions |
| Heating rate, 5–100 °C/min | 5–10 °C/min | 5 °C/min | Higher heating rate (>20 °C/min) cause higher thermal gradient within the sample |
| Gas environment, 0–75 % O2 | 5–75 % O2 | Air or close to air (10–20% O2) | 0 % O2, no combustion for C |
| Sample gas flow rate, mL/min | 25–100 mL/min | 60 mL/min | Low flow can impact on combustion and results via lowering O2 ratio after mixing with the balance gas |
| TGA instrument design (furnace and thermobalance) | Vertical or horizontal | Vertical or horizontal | Insignificant impact. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losic, D.; Farivar, F.; Yap, P.L. Refining and Validating Thermogravimetric Analysis (TGA) for Robust Characterization and Quality Assurance of Graphene-Related Two-Dimensional Materials (GR2Ms). C 2024, 10, 30. https://doi.org/10.3390/c10020030
Losic D, Farivar F, Yap PL. Refining and Validating Thermogravimetric Analysis (TGA) for Robust Characterization and Quality Assurance of Graphene-Related Two-Dimensional Materials (GR2Ms). C. 2024; 10(2):30. https://doi.org/10.3390/c10020030
Chicago/Turabian StyleLosic, Dusan, Farzaneh Farivar, and Pei Lay Yap. 2024. "Refining and Validating Thermogravimetric Analysis (TGA) for Robust Characterization and Quality Assurance of Graphene-Related Two-Dimensional Materials (GR2Ms)" C 10, no. 2: 30. https://doi.org/10.3390/c10020030
APA StyleLosic, D., Farivar, F., & Yap, P. L. (2024). Refining and Validating Thermogravimetric Analysis (TGA) for Robust Characterization and Quality Assurance of Graphene-Related Two-Dimensional Materials (GR2Ms). C, 10(2), 30. https://doi.org/10.3390/c10020030