Acyclic Arylamine-Based Ionophores as Potentiometric Sensors for Zn2+ and Ni2+ Ions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Potentiometric Studies
2.1.1. Determination of Binding Constants
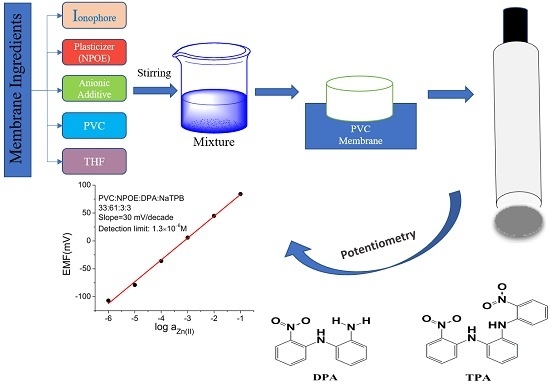
2.1.2. Membrane Optimization
2.1.3. Calibration Curve, Response Time, and Life Time of the Electrode
2.1.4. Effect of pH
2.1.5. Effect of Interfering Ions on Potentiometric Selectivity of Electrodes
2.1.6. Applications
2.1.7. Comparison of the Proposed Sensors with Other Electrodes
2.2. Theoretical Studies
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Synthesis of Ionophores
3.3. Potentiometric Studies
3.3.1. Preparation of Electrode
3.3.2. EMF Measurement
3.4. Quantum Chemical Calculations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-based ion-selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors. Chem. Rev. 1998, 98, 1593–1688. [Google Scholar] [CrossRef] [PubMed]
- Pankratova, N.; Cuartero, M.; Jowett, L.A.; Howe, E.N.; Gale, P.A.; Bakker, E.; Crespo, G.A. Fluorinated tripodal receptors for potentiometric chloride detection in biological fluids. Biosens. Bioelectron. 2018, 99, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, M.; Más-Montoya, M.; García, M.S.; Curiel, D.; Ortuño, J.A. New carbazolo [1,2-a] carbazole derivative as ionophore for anion-selective electrodes: Remarkable recognition towards dicarboxylate anions. Talanta 2014, 123, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, M.; Ortuño, J.A.; García, M.S.; Sánchez, G.; Más-Montoya, M.; Curiel, D. Benzodipyrrole derivates as new ionophores for anion-selective electrodes: Improving potentiometric selectivity towards divalent anions. Talanta 2011, 85, 1876–1881. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.A.; Rezayi, M.; Manan, N.S.; Narimani, L.; Rosli, A.N.B.; Alias, Y. A novel potentiometric sensor based on 1,2-Bis (N′-benzoylthioureido) benzene and reduced graphene oxide for determination of lead (II) cation in raw milk. Electrochim. Acta 2015, 165, 221–231. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Mahmudov, K.T.; Pombeiro, A.J. Poly (vinyl) chloride membrane copper-selective electrode based on 1-phenyl-2-(2-hydroxyphenylhydrazo) butane-1,3-dione. J. Hazard. Mater. 2011, 186, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, A.; Radu, A.; Peper, S.; Bakker, E.; Pretsch, E. Rational design of potentiometric trace level ion sensors. A Ag+-selective electrode with a 100 ppt detection limit. Anal. Chem. 2002, 74, 4027–4036. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Abkenar, S.D.; Ganjali, M.R.; Faridbod, F. Determination of zinc (II) ions in waste water samples by a novel zinc sensor based on a new synthesized Schiff's base. Mater. Sci. Eng. C 2011, 31, 428–433. [Google Scholar] [CrossRef]
- Berg, J.M.; Shi, Y. The galvanization of biology: A growing appreciation for the roles of zinc. Science 1996, 271, 1081. [Google Scholar] [CrossRef] [PubMed]
- Kouremenou-Dona, E.; Dona, A.; Papoutsis, J.; Spiliopoulou, C. Copper and zinc concentrations in serum of healthy Greek adults. Sci. Total Environ. 2006, 359, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Goyal, R.N.; Al Khayat, M.; Kumar, P.; Bachheti, N. A new Zn (II)-selective potentiometric sensor based on 4-tert-butylcalix [4] arene in PVC matrix. Talanta 2006, 69, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- István, P.; Benton, J.J. The Hand Book of Trace Elements; St. Lucie Press: St. Lucie, FL, USA, 1997; Volume 124, ISBN 1-88415-34-4. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 1–20. [Google Scholar] [CrossRef]
- Igbum, O.G.; Asemave, K.; Nwadinigwe, C.A.; Akaasah, N.Y. Heavy Metal Analysis of Blends of Methyl Esters Obtained from Four Virgin Tropical Seed Oils. Int. J. Sci. Res. 2013, 2, 463–467. [Google Scholar]
- Barreto, W.J.; Barreto, S.R.G.; Scarminio, I.S.; Ishikawa, D.N.; Soares, M.D.F.; Proença, M.V.B.D. Determination of Ni (II) in metal alloys by spectrophotometry UV-Vis using dopasemiquinone. Quím. Nova 2010, 33, 109–113. [Google Scholar] [CrossRef]
- Bohn, H.L.; McNeal, B.L.; O’ Connor, A.G. Soil Chemistry, 2nd ed.; John Willey & Sons: New York, NY, USA, 1985. [Google Scholar]
- Hernberg, S.; Nikkanen, J.; Mellin, G.; Lilius, H. δ-aminolevulinic acid dehydrase as a measure of lead exposure. Arch. Environ. Health 1970, 21, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Wilhartitz, P.; Dreer, S.; Krismer, R.; Bobleter, O. High performance ultra trace analysis in molybdenum and tungsten accomplished by on-line coupling of ion chromatography with simultaneous ICP-AES. Microchim. Acta 1997, 125, 45–52. [Google Scholar] [CrossRef]
- Kaur, P.; Kaur, S.; Mahajan, A.; Singh, K. Highly selective colorimetric sensor for Zn2+ based on hetarylazo derivative. Inorg. Chem. Commun. 2008, 11, 626–629. [Google Scholar] [CrossRef]
- Gupta, N.R.; Mittal, S.; Kumar, S.; Kumar, S.A. Potentiometric studies of N,N′-Bis(2-dimethylaminoethyl)-N,N′-dimethyl-9,10 anthracene dimethanamine as a chemical sensing material for Zn (II) ions. Mater. Sci. Eng. C 2008, 28, 1025–1030. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, X.; Lv, Q.; Liu, G. The determination of zinc in water by flame atomic absorption spectrometry after its separation and preconcentration by malachite green loaded microcrystalline triphenylmethane. Sep. Purif. Technol. 2007, 55, 76–81. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Z.; Geng, Z.; Kang, J.; Gao, J. 2,4,6-tri (3,5-Dimethylpyrazoyl)-1,3,5-triazine modified carbon paste electrode for trace Cobalt (II) determination by differential pulse anodic stripping voltammetry. Talanta 2000, 52, 411–416. [Google Scholar] [CrossRef]
- Fakhari, A.R.; Shamsipur, M.; Ghanbari, K. Zn (II)-selective membrane electrode based on tetra (2-aminophenyl) porphyrin. Anal. Chim. Acta 2002, 460, 177–183. [Google Scholar] [CrossRef]
- Shamsipur, M.; Rouhani, S.; Ganjali, M.R.; Sharghi, H.; Eshghi, H. Zinc-selective membrane potentiometric sensor based on a recently synthesized benzo-substituted macrocyclic diamide. Sens. Actuators B 1999, 59, 30–34. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Jakob, A.; Lang, H. A zinc-selective electrode based on N,N′-bis(acetylacetone) ethylenediimine. Sens. Actuators B 2006, 114, 812–818. [Google Scholar] [CrossRef]
- Gupta, V.K.; Al Khayat, M.; Minocha, A.K.; Kumar, P. Zinc (II)-selective sensors based on dibenzo-24-crown-8 in PVC matrix. Anal. Chim. Acta 2005, 532, 153–158. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, A.; Mangla, R. Protoporphyrin IX dimethyl ester as active material in PVC matrix membranes for the fabrication of zinc (II) selective sensor. Sens. Actuators B 2001, 76, 617–623. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, A.K.; Mangla, R.; Kumar, P. A new Zn2+-selective sensor based on 5,10,15,20-tetraphenyl-21H,23H-porphine in PVC Matrix. Electroanalysis 2001, 13, 1036–1040. [Google Scholar] [CrossRef]
- Gupta, V.K. A PVC-based 12-crown-4 membrane potentiometric sensor for zinc (II) ions. Sens. Actuators B 1999, 55, 195–200. [Google Scholar] [CrossRef]
- Jain, A.K.; Sondhi, S.M.; Rajvanshi, S. A PVC based hematoporphyrin IX membrane potentiometric sensor for zinc (II). Electroanalysis 2002, 14, 293–296. [Google Scholar] [CrossRef]
- Rao, G.N.; Srivastava, S.; Srivastava, S.K.; Singh, M. Chelating ion-exchange resin membrane sensor for nickel (II) ions. Talanta 1996, 43, 1821–1825. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, A.K.; Singh, L.P.; Khurana, U. Porphyrins as carrier in PVC based membrane potentiometric sensors for nickel (II). Anal. Chim. Acta 1997, 355, 33–41. [Google Scholar] [CrossRef]
- Jain, A.K.; Gupta, V.K.; Singh, R.D.; Khurana, U.; Singh, L.P. Nickel (II)-selective sensors based on heterogeneous membranes of macrocyclic compounds. Sens. Actuators B 1997, 40, 15–20. [Google Scholar] [CrossRef]
- Gupta, V.K.; Prasad, R.; Kumar, P.; Mangla, R. New nickel (II) selective potentiometric sensor based on 5,7,12,14-tetramethyldibenzotetraazaannulene in a poly (vinyl chloride) matrix. Anal. Chim. Acta 2000, 420, 19–27. [Google Scholar] [CrossRef]
- Mazloum, M.; Niassary, M.S.; Amini, M.K. Pentacyclooctaaza as a neutral carrier in coated-wire ion-selective electrode for nickel (II). Sens. Actuators B 2002, 82, 259–264. [Google Scholar] [CrossRef]
- Singh, A.K.; Jain, A.K.; Saxena, P.; Mehtab, S. Zn (II)-Selective Membrane Electrode Based on Tetraazamacrocycle [Bzo2Me2Ph2(16)hexaeneN4]. Electroanalysis 2006, 18, 1186–1192. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.K.; Jain, A.K. Electrochemical sensors for the determination of Zn2+ ions based on pendant armed macrocyclic ligand. Electrochim. Acta 2011, 56, 5386–5395. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Zamani, H.A.; Norouzi, P.; Adib, M.; Rezapour, M.; Aceedy, M. Zn2+ PVC-based membrane sensor based on 3-[(2-furylmethylene)amino]-2-thioxo-1,3-thiazolidin-4-one. Bull. Korean Chem. Soc. 2005, 26, 579–584. [Google Scholar] [CrossRef]
- Jain, A.K.; Gupta, V.K.; Ganeshpure, P.A.; Raisoni, J.R. Ni (II)-selective ion sensors of salen type Schiff base chelates. Anal. Chim. Acta 2005, 553, 177–184. [Google Scholar] [CrossRef]
- Kumar, K.G.; Poduval, R.; Augustine, P.; John, S.; Saraswathyamma, B. A PVC plasticized sensor for Ni (II) ion based on a simple ethylenediamine derivative. Anal. Sci. 2006, 22, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.P.; Bhatnagar, J.M. PVC based selective sensors for Ni2+ ions using carboxylated and methylated porphine. Sensors 2003, 3, 393–403. [Google Scholar] [CrossRef]
- Mousavi, M.F.; Alizadeh, N.; Shamsipur, M.; Zohari, N. A new PVC-based 1,10-dibenzyl-1,10-diaza-18-crown-6 selective electrode for detecting nickel (II) ion. Sens. Actuators B 2000, 66, 98–100. [Google Scholar] [CrossRef]
- Gupta, V.K.; Prasad, R.; Kumar, A. Dibenzocyclamnickel (II) as ionophore in PVC-matrix for Ni2+-selective sensor. Sensors 2000, 2, 384–396. [Google Scholar] [CrossRef]
- Qin, Y.; Mi, Y.; Bakker, E. Determination of complex formation constants of 18 neutral alkali and alkaline earth metal ionophores in poly (vinyl chloride) sensing membranes plasticized with bis (2-ethylhexyl) sebacate and o-nitrophenyloctylether. Anal. Chim. Acta 2000, 421, 207–220. [Google Scholar] [CrossRef]
- Mi, Y.; Bakker, E. Determination of complex formation constants of lipophilic neutral ionophores in solvent polymeric membranes with segmented sandwich membranes. Anal. Chem. 1999, 71, 5279–5287. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E. Selectivity of liquid membrane ion-selective electrodes. Electroanalysis 1997, 9, 7–12. [Google Scholar] [CrossRef]
- Bereczki, R.; Takács, B.; Langmaier, J.; Neely, M.; Gyurcsányi, R.E.; Tóth, K.; Nagy, G.; Lindner, E. How to assess the limits of ion-selective electrodes: Method for the determination of the ultimate span, response range, and selectivity coefficients of neutral carrier-based cation selective electrodes. Anal. Chem. 2006, 78, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.P.; Lindner, E. Recommendations for nomenclature of ionselective electrodes (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Lindner, E.; Umezawa, Y. Performance evaluation criteria for preparation and measurement of macro-and microfabricated ion-selective electrodes (IUPAC Technical Report). Pure Appl. Chem. 2008, 80, 85–104. [Google Scholar] [CrossRef]
- Umezawa, Y.; Bühlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes. Part I. Inorganic cations (technical report). Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Ma, D.; Cai, Q.; Zhang, H. Mild method for Ullmann coupling reaction of amines and aryl halides. Org. Lett. 2003, 5, 2453–2455. [Google Scholar] [CrossRef] [PubMed]
- Craggs, A.; Moody, G.J.; Thomas, J.D.R. PVC matrix membrane ion-selective electrodes. Construction and laboratory experiments. J. Chem. Educ. 1974, 51, 541. [Google Scholar] [CrossRef]
- Kielland, J. Individual activity coefficients of ions in aqueous solutions. J. Am. Chem. Soc. 1937, 59, 1675–1678. [Google Scholar] [CrossRef]









| Membrane Electrode No. | PVC (Wt %) | Plasticizer (Wt %) | Ionophore (Wt %) | NaTPB (Wt %) | Slope, mV/Decade | Detection Limit, (M) (Zn2+) |
|---|---|---|---|---|---|---|
| 1 | 33 | 63 | 1 | 3 | 20 | 1.0 × 10−5 |
| 2 | 33 | 64 | 2 | 1 | 21 | 8.2 × 10−5 |
| 3 | 33 | 61 | 3 | 3 | 30 | 1.3 × 10−6 |
| 4 | 34 | 61 | 2 | 3 | 21 | 3.2 × 10−5 |
| 5 | 33 | 60 | 4 | 3 | 36 | 6.3 × 10−5 |
| 6 | 32 | 60 | 5 | 3 | 33 | 1.2 × 10−5 |
| 7 | 32 | 59 | 6 | 3 | 20 | 3.6 × 10−5 |
| Membrane Electrode No. | Ionophore (% w/w) | PVC (% w/w) | NPOE (% w/w) | NaTPB (% w/w) | Slope, mV/decade | Detection Limit, (M) (Ni2+) |
|---|---|---|---|---|---|---|
| 1 | - | 33 | 67 | - | - | - |
| 2 | - | 33 | 65 | 2 | - | - |
| 3 | 1 | 33 | 65 | 1 | 14.3 | 2.4 × 10−4 |
| 4 | 1 | 32 | 65 | 2 | 15.2 | 7.1 × 10−4 |
| 5 | 2 | 33 | 64 | 1 | 20.8 | 6.8 × 10−4 |
| 6 | 2 | 32 | 65 | 1 | 19.8 | 2.7 × 10−4 |
| 7 | 2 | 32 | 64 | 2 | 21.2 | 8.2 × 10−5 |
| 8 | 2 | 33 | 63 | 2 | 21.8 | 7.6 × 10−5 |
| 9 | 3 | 32 | 62 | 3 | 30.0 | 2.8 × 10−6 |
| 10 | 3 | 33 | 61 | 3 | 26.5 | 9.2 × 10−6 |
| 11 | 3 | 33 | 63 | 1 | 25.8 | 1.2 × 10−5 |
| 12 | 4 | 33 | 60 | 3 | 37.0 | 4.3 × 10−6 |
| 13 | 4 | 32 | 61 | 3 | 35.2 | 3.8 × 10−5 |
| 14 | 5 | 32 | 61 | 2 | 34.0 | 1.2 × 10−5 |
| 15 | 5 | 32 | 60 | 3 | 33.2 | 8.7 × 10−5 |
| Metal Ion | log KZn2+,B | Metal Ion | log KZn2+,B |
|---|---|---|---|
| K+ | −1.4 | Ni2+ | −2.0 |
| Ca2+ | −2.7 | Cu2+ | −2.5 |
| Na+ | −1.9 | Sr2+ | −2.3 |
| Mg2+ | −2.1 | Ba2+ | −3.1 |
| Pb2+ | −2.4 | Cd2+ | −1.9 |
| Fe2+ | −2.8 | Co2+ | −2.7 |
| Metal Ion | log KNi2+,B | Metal Ion | log KNi2+,B |
|---|---|---|---|
| Na+ | −1.0 | Pb2+ | −2.6 |
| Sr2+ | −3.1 | Fe2+ | −3.2 |
| Cu2+ | −1.9 | Ca2+ | −3.1 |
| Cd2+ | −2.9 | Ba2+ | −3.5 |
| Mg2+ | −2.8 | Zn2+ | −2.1 |
| Co2+ | −3.1 | Mn2+ | −3.2 |
| S. No. | Ionophore | Detection Limit (M) | pH Range | Slope (mV/Decade) | Ref |
|---|---|---|---|---|---|
| 1 | [Bzo2Me2Ph2(16)hexaeneN4] | 2.24 × 10−6 | 2.5–8.5 | 28.5 | [36] |
| 2 | 6,7:14,15-Bzo2-10,11-(4-methylbenzene)-[15]-6,8,12,14-tetraene-9,12-N2-1,5-O2 | 3.3 × 10−7 | 3.0–8.0 | 28.8 | [37] |
| 3 | Dibenzo-24-crown-8 | 9.2 × 10−5 | 4.8–6.2 | 29.0 | [26] |
| 4 | 3-[(2-Furylmethylene)amino]-2-thioxo-1,3-thiazolidin-4-one | 8.5 × 10−7 | 3.0–7.0 | 29.3 | [38] |
| 5 | N,N-Bis(acetylacetone)ethylenediamine | 8.9 × 10−5 | 3.2–7.1 | 30.0 | [25] |
| 6 | This work (DPA) | 1.3 × 10−6 | 3.6–9.3 | 30.0 | - |
| S. No. | Ionophore | Detection Limit (M) | pH Range | Slope (mV/decade) | Ref |
|---|---|---|---|---|---|
| 1 | Hexamethyl-1,4,8,11-tetraazacyclotetradeca-4,11-diene diperchlorate | 1.0 × 10−5 | 1.7–5.4 | Near-Nernstian | [33] |
| 2 | [Me4Bzo2TAA] | 7.9 × 10−6 | 2.7–7.6 | 30 | [34] |
| 3 | N-(2-hydroxybenzylidene)-N-(2-picolyl)ethylenediamine | 3.2 × 10−6 | 2.2–5.9 | 29 | [39] |
| 4 | N1,N2-bis((naphthalen-1-yl)-methylene)ethane-1,2-diamine | 1.3 × 10−6 | 3.6–7.4 | 29.9 | [40] |
| 5 | 2,3,7,8,12,13,17,18-octamethyl-21H, 23H-porphine | 1.0 × 10−5 | 2–7 | 29 | [41] |
| 6 | 1,10-dibenzyl-1,10-diaza-18-crown-6 | 2.0 × 10−5 | 4–8 | Nernstian | [42] |
| 7 | 5,7,8,14-tetramethyldibenzo[b,i]-1,4,8,11-tetrazacyclo tetradecane | 7.0 × 10−6 | 2–7.6 | 29.8 | [43] |
| 8 | This work (TPA) | 2.8 × 10−6 | 4.2–9.2 | 30.0 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, H.; Chhibber, M.; Mittal, S.K. Acyclic Arylamine-Based Ionophores as Potentiometric Sensors for Zn2+ and Ni2+ Ions. C 2017, 3, 34. https://doi.org/10.3390/c3040034
Kaur H, Chhibber M, Mittal SK. Acyclic Arylamine-Based Ionophores as Potentiometric Sensors for Zn2+ and Ni2+ Ions. C. 2017; 3(4):34. https://doi.org/10.3390/c3040034
Chicago/Turabian StyleKaur, Harpreet, Manmohan Chhibber, and Susheel K. Mittal. 2017. "Acyclic Arylamine-Based Ionophores as Potentiometric Sensors for Zn2+ and Ni2+ Ions" C 3, no. 4: 34. https://doi.org/10.3390/c3040034
APA StyleKaur, H., Chhibber, M., & Mittal, S. K. (2017). Acyclic Arylamine-Based Ionophores as Potentiometric Sensors for Zn2+ and Ni2+ Ions. C, 3(4), 34. https://doi.org/10.3390/c3040034