A New Approach of Fabricating Graphene Nanoplates@Natural Rubber Latex Composite and Its Characteristics and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Graphene Nanoplatelets
2.3. Fabrication of GNPs@Natural Rubber Composite
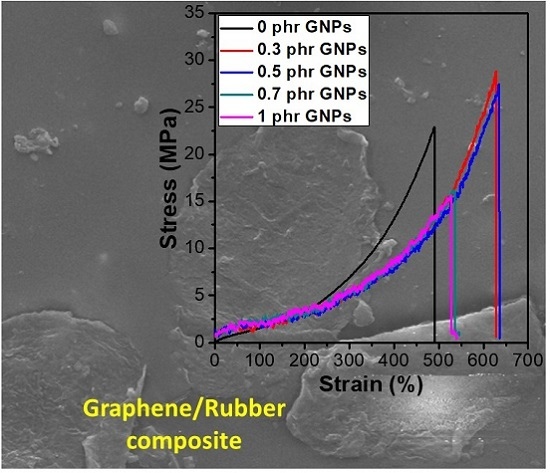
2.4. Sample Preparation for Measurement of Tensile and Tear Strength
2.5. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, K.; Nizami, S.S.; Raza, Z.N.; Mahmood, K. Effect of micro-sized marble sludge on physical properties of natural rubber composites. Chem. Ind. Chem. Eng. Q. 2013, 19, 281–293. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Fahim, I.S.; Elhaggar, S.M.; Elayat, H. Experimental investigation of natural fiber reinforced polymers. Mater. Sci. Appl. 2012, 3, 59–66. [Google Scholar] [CrossRef]
- Gangopadhyay, R.; De, A. Conducting polymer nanocomposites: A brief overview. Chem. Mater. 2000, 12, 608–622. [Google Scholar] [CrossRef]
- Bryning, M.B.; Islam, M.F.; Kikkawa, J.M.; Yodh, A.G. Very low conductivity threshold in bulk isotropic single-walled carbon nanotube-epoxy composites. Adv. Mater. 2005, 17, 1186–1191. [Google Scholar] [CrossRef]
- Rajan, V.; Dierkes, W.K.; Joseph, R.; Noordermeer, J.W. Science and technology of rubber reclamation with special attention to NR-based waste latex products. Prog. Polym. Sci. 2006, 31, 811–834. [Google Scholar] [CrossRef]
- Peng, Z.; Kong, L.X.; Li, S.-D.; Chen, Y.; Huang, M.F. Self-assembled natural rubber/silica nanocomposites: Its preparation and characterization. Compos. Sci. Technol. 2007, 67, 3130–3139. [Google Scholar] [CrossRef]
- Peng, Z.; Feng, C.; Luo, Y.; Li, Y.; Kong, L. Self-assembled natural rubber/multi-walled carbon nanotube composites using latex compounding techniques. Carbon 2010, 48, 4497–4503. [Google Scholar] [CrossRef]
- George, G.; Sisupal, S.B.; Tomy, T.; Pottammal, B.A.; Kumaran, A.; Suvekbala, V.; Gopimohan, R.; Sivaram, S.; Ragupathy, L. Thermally conductive thin films derived from defect free graphene-natural rubber latex nanocomposite: Preparation and properties. Carbon 2017, 119, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Tang, Y.; Yao, L.; Yang, F.; Fang, Q.; Hui, D. Fabrication of graphene/natural rubber nanocomposites with high dynamic properties through convenient mechanical mixing. Compos. Part B Eng. 2017, 112, 1–7. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.A.; Grigorieva, I.; Firsov, A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Bunch, J.S.; Van Der Zande, A.M.; Verbridge, S.S.; Frank, I.W.; Tanenbaum, D.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Electromechanical resonators from graphene sheets. Science 2007, 315, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Katsnelson, M.I. Graphene: Carbon in two dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Kopelevich, Y.; Esquinazi, P. Graphene physics in graphite. Adv. Mater. 2007, 19, 4559–4563. [Google Scholar] [CrossRef]
- Morozov, S.; Novoselov, K.; Katsnelson, M.; Schedin, F.; Elias, D.; Jaszczak, J.; Geim, A. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [PubMed]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef] [PubMed]
- La, D.D.; Rananaware, A.; Salimimarand, M.; Bhosale, S.V. Well-dispersed assembled porphyrin nanorods on graphene for the enhanced photocatalytic performance. ChemistrySelect 2016, 1, 4430–4434. [Google Scholar] [CrossRef]
- La, D.D.; Hangarge, R.V.; Bhosale, S.V.; Ninh, H.D.; Jones, L.A.; Bhosale, S.V. Arginine-Mediated Self-Assembly of Porphyrin on Graphene: A Photocatalyst for Degradation of Dyes. Appl. Sci. 2017, 7, 643. [Google Scholar] [CrossRef]
- La, D.D.; Nguyen, T.A.; Jones, L.A.; Bhosale, S.V. Graphene-Supported Spinel CuFe2O4 Composites: Novel adsorbents for arsenic removal in aqueous media. Sensors 2017, 17, 1292. [Google Scholar] [CrossRef] [PubMed]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Revaprasadu, N.; Bhosale, S.V. Fabrication of a Graphene@TiO2@Porphyrin hybrid material and its photocatalytic properties under simulated sunlight irradiation. ChemistrySelect 2017, 2, 3329–3333. [Google Scholar] [CrossRef]
- La, D.D.; Thi, H.P.N.; Nguyen, T.A.; Bhosale, S. Effective removal of Pb (II) using Graphene@Ternary oxides composite as adsorbent in aqueous media. New J. Chem. 2017, 41, 14627–14634. [Google Scholar] [CrossRef]
- La, D.D.; Patwari, J.M.; Jones, L.A.; Antolasic, F.; Bhosale, S.V. Fabrication of a GNP/Fe–Mg Binary Oxide Composite for Effective Removal of Arsenic from Aqueous Solution. ACS Omega 2017, 2, 218–226. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Wu, J.; Huang, G.; Li, H.; Tang, M.; Fu, X. Enhanced mechanical properties of graphene/natural rubber nanocomposites at low content. Polym. Int. 2014, 63, 1674–1681. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H.; Yu, Z.-Z.; Koratkar, N. Enhanced mechanical properties of nanocomposites at low graphene content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Korattanawittaya, S.; Petcharoen, K.; Sangwan, W.; Tangboriboon, N.; Wattanakul, K.; Sirivat, A. Durable compliant electrode based on graphene and natural rubber. Polym. Eng. Sci. 2017, 57, 129–136. [Google Scholar] [CrossRef]
- Zhan, Y.; Wu, J.; Xia, H.; Yan, N.; Fei, G.; Yuan, G. Dispersion and exfoliation of graphene in rubber by an Ultrasonically-Assisted latex mixing and in situ reduction process. Macromol. Mater. Eng. 2011, 296, 590–602. [Google Scholar] [CrossRef]
- Stanier, D.; Patil, A.; Sriwong, C.; Rahatekar, S.; Ciambella, J. The reinforcement effect of exfoliated graphene oxide nanoplatelets on the mechanical and viscoelastic properties of natural rubber. Compos. Sci. Technol. 2014, 95, 59–66. [Google Scholar] [CrossRef]
- Potts, J.R.; Shankar, O.; Du, L.; Ruoff, R.S. Processing-morphology-property relationships and composite theory analysis of reduced graphene oxide/natural rubber nanocomposites. Macromolecules 2012, 45, 6045–6055. [Google Scholar] [CrossRef]
- Potts, J.R.; Shankar, O.; Murali, S.; Du, L.; Ruoff, R.S. Latex and two-roll mill processing of thermally-exfoliated graphite oxide/natural rubber nanocomposites. Compos. Sci. Technol. 2013, 74, 166–172. [Google Scholar] [CrossRef]
- La, M.D.D.; Bhargava, S.; Bhosale, S.V. Improved and a simple approach for mass production of graphene nanoplatelets material. ChemistrySelect 2016, 1, 949–952. [Google Scholar] [CrossRef]
- Movahed, S.O.; Ansarifar, A.; Mirzaie, F. Effect of various efficient vulcanization cure systems on the compression set of a nitrile rubber filled with different fillers. J. Appl. Polym. Sci. 2015, 132, 41512. [Google Scholar] [CrossRef]
- Schopp, S.; Thomann, R.; Ratzsch, K.F.; Kerling, S.; Altstädt, V.; Mülhaupt, R. Functionalized graphene and carbon materials as components of Styrene-Butadiene rubber nanocomposites prepared by aqueous dispersion blending. Macromol. Mater. Eng. 2014, 299, 319–329. [Google Scholar] [CrossRef]








| Samples | Rubber | GNPs@Rubber |
|---|---|---|
| Compositions | 100 | 100 |
| ZnO | 5 | 5 |
| Acid Stearic | 2 | 2 |
| N-tert-butyl-2-benzothiazolesulphenamide | 0.7 | 0.7 |
| Sulfur | 2.25 | 2.25 |
| GNPs Content, phr | 0 | 0.3 | 0.5 | 0.7 | 1 | |
|---|---|---|---|---|---|---|
| Properties | ||||||
| Stress at 300% elongation, MPa | 7 | 5 | 5 | 5 | 5 | |
| Strain at break, % | 22.5 | 30.0 | 29.2 | 18.3 | 17.9 | |
| Tensile strength, MPa | 22.2 | 29.5 | 28.3 | 18.2 | 17.6 | |
| Elongation at break, % | 5.64 | 19.60 | 18.82 | 10.43 | 11.21 | |
| Tear strength, kN/m | 21.3 | 39.5 | 35.5 | 31.2 | 33.6 | |
| Compression set, % | 6.72 | 8.45 | 11.33 | 15.81 | 5.24 | |
| Abrasion loss, g/cycle | 0.280 | 0.041 | 0.031 | 0.025 | 0.021 | |
| Shore hardness, phr | 40.0 | 41.9 | 40.6 | 41.3 | 41.3 | |
| Shore rebound, % | 76.92 | 48.62 | 82.05 | 80.77 | 80.77 | |
| Graphene Contents (phr) | Abrasion Loss, g/Cycle | Stress at 300% Elongation, MPa | Strain at Break, % | Tensile Strength, MPa | Elongation at Break, % | Shore Hardness, phr | Reference |
|---|---|---|---|---|---|---|---|
| 2 | - | - | - | 20 | - | - | [10] |
| 25 | - | break | - | 9.2 | 260 | 69.7 | [34] |
| 5 | - | 1.28 | 8.96 | 10.5 | - | - | [31] |
| 0.3 | 0.041 | 5 | 30.0 | 29.5 | 19.60 | 41.9 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La, D.D.; Nguyen, T.A.; Quoc, V.D.; Nguyen, T.T.; Nguyen, D.A.; Pham Duy, L.N.; Trung, N.P.; Bhosale, S.V. A New Approach of Fabricating Graphene Nanoplates@Natural Rubber Latex Composite and Its Characteristics and Mechanical Properties. C 2018, 4, 50. https://doi.org/10.3390/c4030050
La DD, Nguyen TA, Quoc VD, Nguyen TT, Nguyen DA, Pham Duy LN, Trung NP, Bhosale SV. A New Approach of Fabricating Graphene Nanoplates@Natural Rubber Latex Composite and Its Characteristics and Mechanical Properties. C. 2018; 4(3):50. https://doi.org/10.3390/c4030050
Chicago/Turabian StyleLa, Duong Duc, Tuan Anh Nguyen, Viet Do Quoc, Tham Thi Nguyen, Duy Anh Nguyen, Linh Nguyen Pham Duy, Nghia Phan Trung, and Sheshanath V. Bhosale. 2018. "A New Approach of Fabricating Graphene Nanoplates@Natural Rubber Latex Composite and Its Characteristics and Mechanical Properties" C 4, no. 3: 50. https://doi.org/10.3390/c4030050
APA StyleLa, D. D., Nguyen, T. A., Quoc, V. D., Nguyen, T. T., Nguyen, D. A., Pham Duy, L. N., Trung, N. P., & Bhosale, S. V. (2018). A New Approach of Fabricating Graphene Nanoplates@Natural Rubber Latex Composite and Its Characteristics and Mechanical Properties. C, 4(3), 50. https://doi.org/10.3390/c4030050