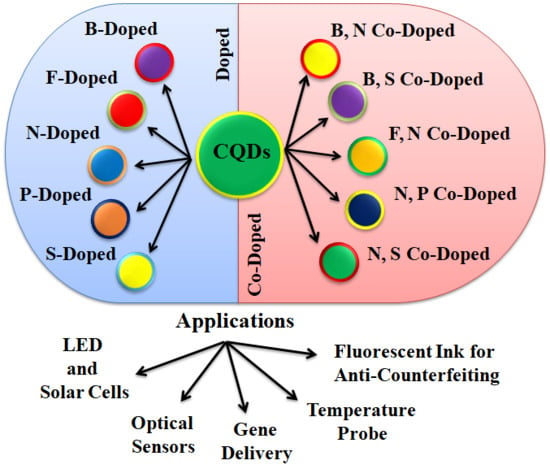
Recent Advancements in Doped/Co-Doped Carbon Quantum Dots for Multi-Potential Applications
Abstract
:1. Introduction
2. Carbon Quantum Dots (CQDs)
2.1. Un-Doped CQDs
2.2. Doped CQDs
2.2.1. B-Doped CQDs
2.2.2. F-Doped CQDs
2.2.3. N-Doped CQDs
2.2.4. P-Doped CQDs
2.2.5. S-Doped CQDs
2.3. Co-Doped CQDs
2.3.1. B, N Co-Doped CQDs
2.3.2. B, S Co-Doped CQDs
2.3.3. F, N Co-Doped CQDs
2.3.4. N, P Co-Doped CQDs
2.3.5. N, S Co-Doped CQDs
2.3.6. B, F, N, P, and/or S Co-Doped CQDs
3. Applications
3.1. Electrical/Electronics
3.1.1. Light Emitting Diodes
3.1.2. Solar Cells
3.2. Fluorescent Ink for Anti-Counterfeiting
3.3. Optical Sensors
3.3.1. Sensing of Metal Ions
Iron (Fe3+) Ions
Mercury (Hg2+) Ions
Chromium (Cr) Ions
Molecular Logic Gates
3.3.2. Detection of Drugs
Methotrexate
Curcumin
3.3.3. Detection of Pesticides and Fungicides
3.4. Gene Delivery
3.5. Temperature Probe
4. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon Dots for Multiphoton Bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef]
- Yang, S.-T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.-P. Carbon dots for optical imaging in vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef]
- Liu, H.; Ye, T.; Mao, C. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chem. Int. Ed. 2007, 46, 6473–6475. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Stassinopoulos, A.; Anglos, D.; Zboril, R.; Karakassides, M.; Giannelis, E.P. Surface functionalized carbogenic quantum dots. Small 2008, 4, 455–458. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Stassinopoulos, A.; Anglos, D.; Zboril, R.; Georgakilas, V.; Giannelis, E.P. Photoluminescent carbogenic dots. Chem. Mater. 2008, 20, 4539–4541. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Liu, S.; Koynov, K.; Knoll, W.; Li, Q. An Aqueous Route to Multicolor Photoluminescent Carbon Dots Using Silica Spheres as Carriers. Angew. Chem. Int. Ed. 2009, 48, 4598–4601. [Google Scholar] [CrossRef]
- Ray, S.C.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent Carbon Nanoparticles: Synthesis, Characterization, and Bioimaging Application. J. Phys. Chem. C 2009, 113, 18546–18551. [Google Scholar] [CrossRef]
- Tian, L.; Ghosh, D.; Chen, W.; Pradhan, S.; Chang, X.; Chen, S. Nanosized Carbon Particles From Natural Gas Soot. Chem. Mater. 2009, 21, 2803–2809. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Li, Y.; Wang, Z.; Yang, F.; Yang, X. Microwave synthesis of fluorescent carbon nanoparticles with electrochemiluminescence properties. Chem. Commun. 2009, 5118–5120. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Li, Z.; Wu, C.; Yan, X.; Wu, M. Observation of pH-, solvent-, spin-, and excitation-dependent blue photoluminescence from carbon nanoparticles. Chem. Commun. 2010, 46, 3681–3683. [Google Scholar] [CrossRef]
- Wang, F.; Pang, S.; Wang, L.; Li, Q.; Kreiter, M.; Liu, C.Y. One-step synthesis of highly luminescent carbon dots in noncoordinating solvents. Chem. Mater. 2010, 22, 4528–4530. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, C.; Liu, Y. A Novel One-Step Approach to Synthesize Fluorescent Carbon Nanoparticles. Eur. J. Inorg. Chem. 2010, 2010, 4411–4414. [Google Scholar] [CrossRef]
- Yang, Z.-C.; Wang, M.; Yong, A.M.; Wong, S.Y.; Zhang, X.-H.; Tan, H.; Chang, A.Y.; Li, X.; Wang, J. Intrinsically fluorescent carbon dots with tunable emission derived from hydrothermal treatment of glucose in the presence of monopotassium phosphate. Chem. Commun. 2011, 47, 11615–11617. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Liu, Y.; Huang, H.; Lian, S.; Lee, S.T.; Kang, Z. One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon 2011, 49, 605–609. [Google Scholar] [CrossRef]
- Lu, A.H.; Hao, G.P.; Sun, Q. Can carbon spheres be created through the stöber method? Angew. Chem. Int. Ed. 2011, 50, 9023–9025. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835. [Google Scholar] [CrossRef]
- Wang, F.; Xie, Z.; Zhang, B.; Liu, Y.; Yang, W.; Liu, C. Down- and up-conversion luminescent carbon dot fluid: Inkjet printing and gel glass fabrication. Nanoscale 2014, 6, 3818–3823. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, N.; Gong, N.; Wang, H.; Shi, X.; Gu, W.; Ye, L. One-step microwave-assisted polyol synthesis of green luminescent carbon dots as optical nanoprobes. Carbon 2014, 68, 258–264. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Esteves da Silva, J.C.G.; Gonçalves, H.M.R. Analytical and bioanalytical applications of carbon dots. TrAC Trends Anal. Chem. 2011, 30, 1327–1336. [Google Scholar] [CrossRef]
- Dimos, K. Carbon Quantum Dots: Surface Passivation and Functionalization. Curr. Org. Chem. 2016, 20, 682–695. [Google Scholar] [CrossRef]
- Park, Y.; Yoo, J.; Lim, B.; Kwon, W.; Rhee, S.-W. Improving Functionality of Carbon Nanodots: Doping and Surface Functionalization. J. Mater. Chem. A 2016, 4, 11582–11603. [Google Scholar] [CrossRef]
- Das, A.; Snee, P.T. Synthetic Developments of Nontoxic Quantum Dots. ChemPhysChem 2016, 17, 598–617. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.; Liu, C.; Ma, D. White light-emitting devices based on carbon dots’ electroluminescence. Chem. Commun. 2011, 47, 3502. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230. [Google Scholar] [CrossRef]
- Gao, X.; Du, C.; Zhuang, Z.; Chen, W. Carbon quantum dot-based nanoprobes for metal ion detection. J. Mater. Chem. C 2016, 4, 6927–6945. [Google Scholar] [CrossRef]
- Grumezescu, A.M. Nanobiomaterials in Medical Imaging: Applications of Nanobiomaterials; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780323417365. [Google Scholar]
- Guo, Y.; Zhang, L.; Zhang, S.; Yang, Y.; Chen, X.; Zhang, M. Fluorescent carbon nanoparticles for the fluorescent detection of metal ions. Biosens. Bioelectron. 2015, 63, 61–71. [Google Scholar] [CrossRef]
- Roy, P.; Chen, P.C.; Periasamy, A.P.; Chen, Y.N.; Chang, H.T. Photoluminescent carbon nanodots: Synthesis, physicochemical properties and analytical applications. Mater. Today 2015, 18, 447–458. [Google Scholar] [CrossRef]
- Miao, P.; Han, K.; Tang, Y.; Wang, B.; Lin, T.; Cheng, W. Recent advances in carbon nanodots: Synthesis, properties and biomedical applications. Nanoscale 2015, 7, 1586–1595. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Z.; Xu, M.; Xu, M.; Ma, Y.; Zhang, J.; Su, Y.; Su, Y.; Gao, F.; Wei, H.; et al. Controllable Synthesis of Fluorescent Carbon Dots and Their Detection Application as Nanoprobes. Nano-Micro Lett. 2013, 5, 247–259. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, S.-H. Carbon dots: Large-scale synthesis, sensing and bioimaging. Mater. Today 2015, 19, 382–393. [Google Scholar] [CrossRef]
- Ding, C.; Zhu, A.; Tian, Y. Functional surface engineering of C-dots for fluorescent biosensing and in vivo bioimaging. Acc. Chem. Res. 2014, 47, 20–30. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Gao, C.; Wang, E. Applications of carbon quantum dots in electrochemiluminescence: A mini review. Electrochem. Commun. 2014, 48, 151–154. [Google Scholar] [CrossRef]
- Yuan, L.; Dai, J.; Fan, X.; Song, T.; Tao, Y.T.; Wang, K.; Xu, Z.; Zhang, J.; Bai, X.; Lu, P.; et al. Self-cleaning flexible infrared nanosensor based on carbon nanoparticles. ACS Nano 2011, 5, 4007–4013. [Google Scholar] [CrossRef]
- Zhu, L.; Yin, Y.; Wang, C.-F.; Chen, S. Plant leaf-derived fluorescent carbon dots for sensing, patterning and coding. J. Mater. Chem. C 2013, 1, 4925–4932. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, G.; Zhang, W.; Wang, Z.; Cheng, Y.; Dai, Z. Solid phase reaction method for preparation of carbon dots and multi-purpose applications. Sens. Actuators B Chem. 2016, 234, 15–20. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, F.; Li, J.; Zhu, J.-J.; Lu, Y. Fluorescent nanoprobes for sensing and imaging of metal ions: Recent advances and future perspectives. Nano Today 2016, 11, 309–329. [Google Scholar] [CrossRef]
- Du, Y.; Guo, S. Chemically doped fluorescent carbon and graphene quantum dots for bioimaging, sensor, catalytic and photoelectronic applications. Nanoscale 2016, 8, 2532–2543. [Google Scholar] [CrossRef]
- Li, X.; Rui, M.; Song, J.; Shen, Z.; Zeng, H. Carbon and Graphene Quantum Dots for Optoelectronic and Energy Devices: A Review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
- Zuo, J.; Jiang, T.; Zhao, X.; Xiong, X.; Xiao, S.; Zhu, Z. Preparation and Application of Fluorescent Carbon Dots. J. Nanomater. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Long, Y.; Wang, X.; Zhang, H.; Zhu, R.; Liang, L.; Teng, P.; Zheng, H. Hollow luminescent carbon dots for drug delivery. Carbon 2013, 59, 192–199. [Google Scholar] [CrossRef]
- Zhao, A.; Chen, Z.; Zhao, C.; Gao, N.; Ren, J.; Qu, X. Recent advances in bioapplications of C-dots. Carbon 2015, 85, 309–327. [Google Scholar] [CrossRef]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2014, 44, 362–381. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon dots—Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, L.; Wang, N.; Sun, L.; Wang, W.; Liu, W. Surface passivated carbon nanodots prepared by microwave assisted pyrolysis: Effect of carboxyl group in precursors on fluorescence properties. RSC Adv. 2014, 4, 18818. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.-J. Quantum dots for fluorescent biosensing and bio-imaging applications. Analyst 2013, 138, 2506. [Google Scholar] [CrossRef] [PubMed]
- Gnaneshwar, P.V.; Sabarikirishwaran, P. Structural and morphological study of carbon nanoparticles synthesized using oxidation, thermal decomposition and solvo chemical methods. Int. J. ChemTech Res. 2015, 7, 1465–1473. [Google Scholar]
- Li, F.; Yang, D.; Xu, H. Non-Metal-Heteroatom-Doped Carbon Dots: Synthesis and Properties. Chem. A Eur. J. 2019, 25, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, T. Photoluminescence tuning in carbon dots: Surface passivation or/and functionalization, heteroatom doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar] [CrossRef]
- Jana, J.; Pal, T. An account of doping in carbon dots for varied applications. Nat. Resour. Eng. 2018, 2, 5–12. [Google Scholar] [CrossRef]
- Atabaev, T. Doped Carbon Dots for Sensing and Bioimaging Applications: A Minireview. Nanomaterials 2018, 8, 342. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. Chem. Soc. Rev 2015, 44, 4743–4768. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Y.; Tsai, P.; Wang, J.; Chen, X. Metal ions doped carbon quantum dots: Synthesis, physicochemical properties, and their applications. TrAC Trends Anal. Chem. 2018, 103, 87–101. [Google Scholar] [CrossRef]
- Shan, X.; Chai, L.; Ma, J.; Qian, Z.; Chen, J.; Feng, H. B-doped carbon quantum dots as a sensitive fluorescence probe for hydrogen peroxide and glucose detection. Analyst 2014, 139, 2322–2325. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Trivizas, G.; Karakassides, M.A.; Baikousi, M.; Kouloumpis, A.; Gournis, D.; Bakandritsos, A.; Hola, K.; Kozak, O.; Zboril, R.; et al. Green and simple route toward boron doped carbon dots with significantly enhanced non-linear optical properties. Carbon 2015, 83, 173–179. [Google Scholar] [CrossRef]
- Jana, J.; Ganguly, M.; Chandrakumar, K.R.S.; Rao, G.M.; Pal, T. Boron precursor-dependent evolution of differently emitting carbon dots. Langmuir 2017, 33, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Hu, Y.; Li, Y.; Zeng, Q.; Jiang, X.; Cheng, Z. Boron doped carbon dots as a multifunctional fluorescent probe for sorbate and vitamin B12. Microchim. Acta 2019, 186, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Xie, A.; Li, J.; Su, T.; Pan, X.; Dong, W. Large Emission Red-Shift of Carbon Dots by Fluorine Doping and Their Applications for Red Cell Imaging and Sensitive Intracellular Ag+ Detection. J. Phys. Chem. C 2017, 121, 26558–26565. [Google Scholar] [CrossRef]
- Zuo, G.; Xie, A.; Pan, X.; Su, T.; Li, J.; Dong, W. Fluorine-Doped Cationic Carbon Dots for Efficient Gene Delivery. ACS Appl. Nano Mater. 2018, 1, 2376–2385. [Google Scholar] [CrossRef]
- Luo, T.Y.; He, X.; Zhang, J.; Chen, P.; Liu, Y.H.; Wang, H.J.; Yu, X.Q. Photoluminescent F-doped carbon dots prepared by ring-opening reaction for gene delivery and cell imaging. RSC Adv. 2018, 8, 6053–6062. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal Treatment of Grass: A Low-Cost, Green Route to Nitrogen-Doped, Carbon-Rich, Photoluminescent Polymer Nanodots as an Effective Fluorescent Sensing Platform for Label-Free Detection of Cu(II) Ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef]
- Dey, S.; Chithaiah, P.; Belawadi, S.; Biswas, K.; Rao, C.N.R. New methods of synthesis and varied properties of carbon quantum dots with high nitrogen content. J. Mater. Res. 2014, 29, 383–391. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.; Jain, A.; Susan Zhou, H. Aqueous phase synthesis of highly luminescent, nitrogen-doped carbon dots and their application as bioimaging agents. Langmuir 2014, 30, 14270–14275. [Google Scholar] [CrossRef]
- Niu, J.; Gao, H. Synthesis and drug detection performance of nitrogen-doped carbon dots. J. Lumin. 2014, 149, 159–162. [Google Scholar] [CrossRef]
- Qian, Z.; Ma, J.; Shan, X.; Feng, H.; Shao, L.; Chen, J. Highly luminescent N-doped carbon quantum dots as an effective multifunctional fluorescence sensing platform. Chem. A Eur. J. 2014, 20, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Liu, X.; Liu, P.; Wang, Y.; An, T.; Yang, H.; Jing, D.; Zhao, H. Determination of Iodide via Direct Fluorescence Quenching at Nitrogen-Doped Carbon Quantum Dot Fluorophores. Environ. Sci. Technol. Lett. 2013, 1, 87–91. [Google Scholar] [CrossRef]
- Campos, B.B.; Abellán, C.; Zougagh, M.; Jimenez-Jimenez, J.; Rodríguez-Castellón, E.; Esteves da Silva, J.C.G.; Ríos, A.; Algarra, M. Fluorescent chemosensor for pyridine based on N-doped carbon dots. J. Colloid Interface Sci. 2015, 458, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Yao, Y.; Zhou, H.; Zhang, J.; Pang, Z.; Ao, K.; Cai, Y.; Wei, Q. Synthesis of novel nitrogen-doped carbon dots for highly selective detection of iron ion. Nanotechnology 2017, 28, 165502. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Su, H.; Wang, K.; Wong, W.-K.; Zhu, X. Facile synthesis of N-rich carbon quantum dots from porphyrins as efficient probes for bioimaging and biosensing in living cells. Int. J. Nanomed. 2017, 12, 7375–7391. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Li, L.; Jin, W.J. Controlling speciation of nitrogen in nitrogen-doped carbon dots by ferric ion catalysis for enhancing fluorescence. Carbon 2017, 111, 133–141. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, P.; Wang, T.Y.; Kong, J.L.; Xiong, H.M. Nitrogen-doped carbon dots derived from polyvinyl pyrrolidone and their multicolor cell imaging. Nanotechnology 2014, 25, 205604. [Google Scholar] [CrossRef]
- Wang, H.; Gao, P.; Wang, Y.; Guo, J.; Zhang, K.-Q.; Du, D.; Dai, X.; Zou, G. Fluorescently tuned nitrogen-doped carbon dots from carbon source with different content of carboxyl groups. APL Mater. 2015, 3, 086102. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Wang, Y.; Cai, C.; Lin, H. Bright-Yellow-Emissive N-Doped Carbon Dots: Preparation, Cellular Imaging, and Bifunctional Sensing. ACS Appl. Mater. Interfaces 2015, 7, 23231–23238. [Google Scholar] [CrossRef]
- Ji, H.; Zhou, F.; Gu, J.; Shu, C.; Xi, K.; Jia, X. Nitrogen-doped carbon dots as a new substrate for sensitive glucose determination. Sensors 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Li, M.; Huang, J.; Fan, H.; Ding, H.; Huang, K.; Huang, L.; Xu, L.; Xia, J.; Li, S. Eosinophilic nitrogen-doped carbon dots derived from tribute chrysanthemum for label-free detection of Fe3+ ions and hydrazine. J. Taiwan Inst. Chem. Eng. 2017, 78, 247–253. [Google Scholar]
- Jiang, X.; Qin, D.; Mo, G.; Feng, J.; Yu, C.; Mo, W.; Deng, B. Ginkgo leaf-based synthesis of nitrogen-doped carbon quantum dots for highly sensitive detection of salazosulfapyridine in mouse plasma. J. Pharm. Biomed. Anal. 2019, 164, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, X.; Sun, Y. One-step synthesis of self-doped carbon dots with highly photoluminescence as multifunctional biosensors for detection of iron ions and pH. Sens. Actuators B Chem. 2017, 241, 73–79. [Google Scholar] [CrossRef]
- Wu, P.; Li, W.; Wu, Q.; Liu, Y.; Liu, S. Hydrothermal synthesis of nitrogen-doped carbon quantum dots from microcrystalline cellulose for the detection of Fe3+ ions in an acidic environment. RSC Adv. 2017, 7, 44144–44153. [Google Scholar] [CrossRef]
- Hou, J.; Wang, W.; Zhou, T.; Wang, B.; Li, H.; Ding, L. Synthesis and formation mechanistic investigation of nitrogen-doped carbon dots with high quantum yield and yellowish-green fluorescence. Nanoscale 2016, 8, 11185–11193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Rao, L.; Li, Z.; Lu, H.; Yan, C.; Yu, S.; Ding, X.; Yu, B. Rapid synthesis of highly photoluminescent nitrogen-doped carbon quantum dots via a microreactor with foamy copper for the detection of Hg2+ ions. Sens. Actuators B Chem. 2018, 258, 637–647. [Google Scholar] [CrossRef]
- Zheng, M.; Xie, Z.; Qu, D.; Li, D.; Du, P.; Jing, X.; Sun, Z. On-off-on fluorescent carbon dot nanosensor for recognition of chromium(VI) and ascorbic acid based on the inner filter effect. ACS Appl. Mater. Interfaces 2013, 5, 13242–13247. [Google Scholar] [CrossRef] [PubMed]
- Meiling, T.T.; Schürmann, R.; Vogel, S.; Ebel, K.; Nicolas, C.; Milosavljević, A.R.; Bald, I. Photophysics and Chemistry of Nitrogen-Doped Carbon Nanodots with High Photoluminescence Quantum Yield. J. Phys. Chem. C 2018, 122, 10217–10230. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, X.; Ma, J.; Gu, Y.; Qian, Z.; Chen, J.; Feng, H. Facile synthesis of P-doped carbon quantum dots with highly efficient photoluminescence. RSC Adv. 2014, 4, 5465–5468. [Google Scholar] [CrossRef]
- Shi, D.; Yan, F.; Zheng, T.; Wang, Y.; Zhou, X.; Chen, L. P-doped carbon dots act as a nanosensor for trace 2,4,6-trinitrophenol detection and a fluorescent reagent for biological imaging. RSC Adv. 2015, 5, 98492–98499. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, K.; Ghosh, M.; Das, P.K. Amino acid functionalized blue and phosphorous-doped green fluorescent carbon dots as bioimaging probe. RSC Adv. 2015, 5, 65913–65921. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Luo, C.; Wang, Y.; Jiang, C.; Qi, R.; Huang, R.; Travas-sejdic, J.; Peng, H. Aggregation induced red shift emission of phosphorus doped carbon dots. RSC Adv. 2017, 7, 32225–32228. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, S.; Yang, X. Phosphorus-doped carbon dots for sensing both Au (III) and L-methionine. J. Photochem. Photobiol. A Chem. 2018, 365, 178–184. [Google Scholar] [CrossRef]
- Yang, F.; He, X.; Wang, C.; Cao, Y.; Li, Y.; Yan, L.; Liu, M.; Lv, M.; Yang, Y.; Zhao, X.; et al. Controllable and Eco-Friendly Synthesis of P-Riched Carbon Quantum Dots and Its Application for Copper (II) Ion Sensing; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 448, ISBN 8601089739. [Google Scholar]
- Wang, W.; Li, Y.; Cheng, L.; Cao, Z.; Liu, W. Water-soluble and phosphorus-containing carbon dots with strong green fluorescence for cell labeling. J. Mater. Chem. B 2014, 2, 46–48. [Google Scholar] [CrossRef]
- Omer, K.M.; Hassan, A.Q. Chelation-enhanced fluorescence of phosphorus doped carbon nanodots for multi-ion detection. Microchim. Acta 2017, 184, 2063–2071. [Google Scholar] [CrossRef]
- Chandra, S.; Patra, P.; Pathan, S.H.; Roy, S.; Mitra, S.; Layek, A.; Bhar, R.; Pramanik, P.; Goswami, A. Luminescent S-doped carbon dots: An emergent architecture for multimodal applications. J. Mater. Chem. B 2013, 1, 2375–2382. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, J.; Tian, J.; Jia, L.; Yu, J.S. Waste frying oil as a precursor for one-step synthesis of sulfur-doped carbon dots with pH-sensitive photoluminescence. Carbon 2014, 77, 775–782. [Google Scholar] [CrossRef]
- Xu, Q.; Pu, P.; Zhao, J.; Dong, C.; Gao, C.; Chen, Y.; Chen, J.; Liu, Y.; Zhou, H. Preparation of highly photoluminescent sulfur-doped carbon dots for Fe(III) detection. J. Mater. Chem. A 2015, 3, 542–546. [Google Scholar] [CrossRef]
- Dany Rahmayanti, H.; Sulhadi; Aji, M.P. Synthesis of Sulfur-Doped Carbon Dots by Simple Heating Method. Adv. Mater. Res. 2015, 1123, 233–236. [Google Scholar] [CrossRef]
- Yang, H.; Li, F.; Zou, C.; Huang, Q.; Chen, D. Sulfur-doped carbon quantum dots and derived 3D carbon nanoflowers are effective visible to near infrared fluorescent probes for hydrogen peroxide. Microchim. Acta 2017, 184, 2055–2062. [Google Scholar] [CrossRef]
- Travlou, N.A.; Secor, J.; Bandosz, T.J. Highly luminescent S-doped carbon dots for the selective detection of ammonia. Carbon 2017, 114, 544–556. [Google Scholar] [CrossRef]
- Loukanov, A.; Mladenova, P.; Udono, H.; Miskolczy, Z.; Angelov, A.; Biczók, L.; Nakabayashi, S. Sulfur doped fluorescent carbon dots as nanosensors for rapid and sensitive monitoring of calcium in hard water. J. Chem. Technol. Metall. 2018, 53, 473–479. [Google Scholar]
- Pawar, S.P.; Anbhule, P.V.; Naik, V.M.; Kolekar, G.B.; Gunjal, D.B.; Gore, A.H.; Mahanwar, S.T. Quick and low cost synthesis of sulphur doped carbon dots by simple acidic carbonization of sucrose for the detection of Fe3+ ions in highly acidic environment. Diam. Relat. Mater. 2018, 88, 262–268. [Google Scholar]
- Wu, F.; Yang, M.; Zhang, H.; Zhu, S.; Zhu, X.; Wang, K. Facile synthesis of sulfur-doped carbon quantum dots from vitamin B1 for highly selective detection of Fe3+ ion. Opt. Mater. 2018, 77, 258–263. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Xu, L.; Qiao, Y.; Chen, J. Facile Synthesis of N, B-Doped Carbon Dots and Their Application for Multisensor and Cellular Imaging. Ind. Eng. Chem. Res. 2017, 56, 3905–3912. [Google Scholar] [CrossRef]
- Ye, Q.; Yan, F.; Shi, D.; Zheng, T.; Wang, Y.; Zhou, X.; Chen, L. N, B-doped carbon dots as a sensitive fluorescence probe for Hg2+ ions and 2,4,6-trinitrophenol detection for bioimaging. J. Photochem. Photobiol. B Biol. 2016, 162, 1–13. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Cao, F.; Wang, L.; Wang, Z.; Leng, Y. Hydrothermal synthesis of nitrogen and boron doped carbon quantum dots with yellow-green emission for sensing Cr(VI), anti-counterfeiting and cell imaging. RSC Adv. 2017, 7, 48386–48393. [Google Scholar] [CrossRef]
- Huang, S.; Yang, E.; Yao, J.; Liu, Y.; Xiao, Q. Carbon dots doped with nitrogen and boron as ultrasensitive fluorescent probes for determination of α-glucosidase activity and its inhibitors in water samples and living cells. Microchim. Acta 2018, 185, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Li, N.B.; Liu, S.G.; Li, N.; Mo, S.; Xiao, N.; Ju, Y.J.; Luo, H.Q.; Ling, Y. Highly selective detection of p-nitrophenol using fluorescence assay based on boron, nitrogen co-doped carbon dots. Talanta 2018, 184, 184–192. [Google Scholar]
- Xiao, N.; Liu, S.G.; Mo, S.; Yang, Y.Z.; Han, L.; Ju, Y.J.; Li, N.B.; Luo, H.Q. B,N-carbon dots-based ratiometric fluorescent and colorimetric dual-readout sensor for H2O2 and H2O2-involved metabolites detection using ZnFe2O4 magnetic microspheres as peroxidase mimics. Sens. Actuators B Chem. 2018, 273, 1735–1743. [Google Scholar] [CrossRef]
- Tian, T.; He, Y.; Ge, Y.; Song, G. One-pot synthesis of boron and nitrogen co-doped carbon dots as the fluorescence probe for dopamine based on the redox reaction between Cr(VI) and dopamine. Sens. Actuators B Chem. 2017, 240, 1265–1271. [Google Scholar] [CrossRef]
- Zhao, C.; Jiao, Y.; Zhang, L.; Yang, Y. One-step synthesis of S,B co-doped carbon dots and their application for selective and sensitive fluorescence detection of diethylstilbestrol. New J. Chem. 2018, 42, 2857–2864. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, H.; Lai, J.; Peng, X.; Hu, Y.; Gu, W.; Ye, L. Carbon dots with red-shifted photoluminescence by fluorine doping for optical bio-imaging. Carbon 2018, 128, 78–85. [Google Scholar] [CrossRef]
- Omer, K.M.; Tofiq, D.I.; Ghafoor, D.D. Highly photoluminescent label free probe for Chromium (II) ions using carbon quantum dots co-doped with nitrogen and phosphorous. J. Lumin. 2019, 206, 540–546. [Google Scholar] [CrossRef]
- Huang, S.; Yang, E.; Liu, Y.; Yao, J.; Su, W.; Xiao, Q. Low-temperature rapid synthesis of nitrogen and phosphorus dual-doped carbon dots for multicolor cellular imaging and hemoglobin probing in human blood. Sens. Actuators B Chem. 2018, 265, 326–334. [Google Scholar] [CrossRef]
- Liao, S.; Zhu, F.; Zhao, X.; Yang, H.; Chen, X. A reusable P, N-doped carbon quantum dot fluorescent sensor for cobalt ion. Sens. Actuators B Chem. 2018, 260, 156–164. [Google Scholar] [CrossRef]
- Li, H.; Shao, F.Q.; Zou, S.Y.; Yang, Q.J.; Huang, H.; Feng, J.J.; Wang, A.J. Microwave-assisted synthesis of N,P-doped carbon dots for fluorescent cell imaging. Microchim. Acta 2016, 183, 821–826. [Google Scholar] [CrossRef]
- Chandra, S.; Laha, D.; Pramanik, A.; Ray Chowdhuri, A.; Karmakar, P.; Sahu, S.K. Synthesis of highly fluorescent nitrogen and phosphorus doped carbon dots for the detection of Fe3+ ions in cancer cells. Luminescence 2016, 31, 81–87. [Google Scholar] [CrossRef]
- Chandra, S.; Chowdhuri, A.R.; Laha, D.; Sahu, S.K. Fabrication of nitrogen- and phosphorous-doped carbon dots by the pyrolysis method for iodide and iron(III) sensing. Luminescence 2018, 33, 336–344. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, X.; Dong, W.; Zhou, R.; Shuang, S.; Dong, C. Nitrogen and phosphorus dual-doped carbon dots as a label-free sensor for Curcumin determination in real sample and cellular imaging. Talanta 2018, 183, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Omer, K.M.; Tofiq, D.I.; Hassan, A.Q. Solvothermal synthesis of phosphorus and nitrogen doped carbon quantum dots as a fluorescent probe for iron(III). Microchim. Acta 2018, 185, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiao, Y.; Feng, L.; Zhong, Y.; Zuo, G.; Xie, A.; Dong, W. Highly N,P-doped carbon dots: Rational design, photoluminescence and cellular imaging. Microchim. Acta 2017, 184, 2933–2940. [Google Scholar] [CrossRef]
- Yang, Y.; Huo, D.; Wu, H.; Wang, X.; Yang, J.; Bian, M.; Ma, Y.; Hou, C. N, P-doped carbon quantum dots as a fluorescent sensing platform for carbendazim detection based on fluorescence resonance energy transfer. Sens. Actuators B Chem. 2018, 274, 296–303. [Google Scholar] [CrossRef]
- Sun, X.; Brückner, C.; Lei, Y. One-pot and ultrafast synthesis of nitrogen and phosphorus co-doped carbon dots possessing bright dual wavelength fluorescence emission. Nanoscale 2015, 7, 17278–17282. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Chen, Z.; Zhao, Z.; Sun, X.; Zhang, J.; Hou, L.; Yuan, C. Green and Facile Synthesis of Nitrogen and Phosphorus Co-Doped Carbon Quantum Dots towards Fluorescent Ink and Sensing Applications. Nanomaterials 2018, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Yu, B.; Yang, W.; Zhang, X. Phosphorus, and nitrogen co-doped carbon dots as a fluorescent probe for real-time measurement of reactive oxygen and nitrogen species inside macrophages. Biosens. Bioelectron. 2016, 79, 822–828. [Google Scholar] [CrossRef]
- Shamsipur, M.; Molaei, K.; Molaabasi, F.; Hosseinkhani, S.; Alizadeh, N.; Alipour, M.; Moassess, S. One-step synthesis and characterization of highly luminescent nitrogen and phosphorus co-doped carbon dots and their application as highly selective and sensitive nanoprobes for low level detection of uranyl ion in hair and water samples and application. Sens. Actuators B Chem. 2018, 257, 772–782. [Google Scholar] [CrossRef]
- Hu, Q.; Paau, M.C.; Zhang, Y.; Gong, X.; Zhang, L.; Lu, D.; Liu, Y.; Liu, Q.; Yao, J.; Choi, M.M.F. Green synthesis of fluorescent nitrogen/sulfur-doped carbon dots and investigation of their properties by HPLC coupled with mass spectrometry. RSC Adv. 2014, 4, 18065–18073. [Google Scholar] [CrossRef]
- Akhgari, F.; Samadi, N.; Farhadi, K.; Akhgari, M. A green one-pot synthesis of nitrogen and sulfur co-doped carbon quantum dots for sensitive and selective detection of cephalexin. Can. J. Chem. 2017, 95, 641–648. [Google Scholar] [CrossRef]
- Cheng, C.; Xing, M.; Wu, Q. A universal facile synthesis of nitrogen and sulfur co-doped carbon dots from cellulose-based biowaste for fluorescent detection of Fe3+ ions and intracellular bioimaging. Mater. Sci. Eng. C 2019, 99, 611–619. [Google Scholar] [CrossRef]
- Simões, E.F.C.; Leitão, J.M.M.; Esteves da Silva, J.C.G. Sulfur and nitrogen co-doped carbon dots sensors for nitric oxide fluorescence quantification. Anal. Chim. Acta 2017, 960, 117–122. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, X.; Milcovich, G.; Chen, J.; Chen, C.; Feng, J.; Hudson, S.P.; Weng, X.; Ruan, Y. Nitrogen and sulfur co-doped carbon nanodots toward bovine hemoglobin: A fluorescence quenching mechanism investigation. J. Mol. Recognit. 2019, 32, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Weng, B.; Wang, B.; Li, C. Facile synthesis of nitrogen and sulfur co-doped carbon dots and application for Fe(III) ions detection and cell imaging. Sens. Actuators B Chem. 2016, 223, 689–696. [Google Scholar] [CrossRef]
- Zhao, S.; Lan, M.; Zhu, X.; Xue, H.; Ng, T.W.; Meng, X.; Lee, C.S.; Wang, P.; Zhang, W. Green Synthesis of Bifunctional Fluorescent Carbon Dots from Garlic for Cellular Imaging and Free Radical Scavenging. ACS Appl. Mater. Interfaces 2015, 7, 17054–17060. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Wang, P.; Yang, Y.; Wang, Y.; Xu, J.; Wang, Y.; Yu, W.W. Synthesis of Nitrogen and Sulfur Co-doped Carbon Dots from Garlic for Selective Detection of Fe3+. Nanoscale Res. Lett. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Li, J.; Xu, L.; Qiao, Y. One-pot synthesis of nitrogen and sulfur co-doped carbon dots and its application for sensor and multicolor cellular imaging. J. Colloid Interface Sci. 2017, 485, 167–174. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.S.; Xiong, H.M. Nitrogen and sulfur co-doped carbon dots with strong blue luminescence. Nanoscale 2014, 6, 13817–13823. [Google Scholar] [CrossRef]
- Liao, S.; Zhao, X.; Zhu, F.; Chen, M.; Wu, Z.; Song, X.; Yang, H.; Chen, X. Novel S, N-doped carbon quantum dot-based “off-on” fluorescent sensor for silver ion and cysteine. Talanta 2018, 180, 300–308. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-Based Dots Co-doped with Nitrogen and Sulfur for High Quantum Yield and Excitation-Independent Emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef]
- Aswathy, B.; Aparna, R.S.; Anjali Devi, J.S.; Sony, G.; Anjana, R.R.; Lekha, G.M.; Praveen, G.L.; Jayasree, M. S,N-doped carbon dots as a fluorescent probe for bilirubin. Microchim. Acta 2017, 185, 1–11. [Google Scholar]
- Zhu, X.; Wang, J.; Zhu, Y.; Jiang, H.; Tan, D.; Xu, Z.; Mei, T.; Li, J.; Xue, L.; Wang, X. Green emitting N,S-co-doped carbon dots for sensitive fluorometric determination of Fe(III) and Ag(I) ions, and as a solvatochromic probe. Microchim. Acta 2018, 185, 1–10. [Google Scholar] [CrossRef]
- Zhou, W.; Zhuang, J.; Li, W.; Hu, C.; Lei, B.; Liu, Y. Towards efficient dual-emissive carbon dots through sulfur and nitrogen co-doped. J. Mater. Chem. C 2017, 5, 8014–8021. [Google Scholar] [CrossRef]
- Bose, M.; Das, N.C.; Ganguly, S.; Mondal, S.; Das, A.K.; Das, P.; Banerjee, S. Heteroatom doped photoluminescent carbon dots for sensitive detection of acetone in human fluids. Sens. Actuators B Chem. 2018, 266, 583–593. [Google Scholar]
- Wei, Z.; Wang, B.; Liu, Y.; Liu, Z.; Zhang, H.; Zhang, S.; Chang, J.; Lu, S. Green synthesis of nitrogen and sulfur co-doped carbon dots from Allium fistulosum for cell imaging. New J. Chem. 2019, 43, 718–723. [Google Scholar] [CrossRef]
- Xu, Y.; Li, D.; Liu, M.; Niu, F.; Liu, J.; Wang, E. Enhanced-quantum yield sulfur/nitrogen co-doped fluorescent carbon nanodots produced from biomass Enteromorpha prolifera: Synthesis, posttreatment, applications and mechanism study. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Zhao, C.; Jiao, Y.; Gao, Z.; Yang, Y.; Li, H. N, S co-doped carbon dots for temperature probe and the detection of tetracycline based on the inner filter effect. J. Photochem. Photobiol. A Chem. 2018, 367, 137–144. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Yang, H.; Zhao, K.; Li, J.; Deng, A. Fluorescent nitrogen and sulfur co-doped carbon dots from casein and their applications for sensitive detection of Hg2+ and biothiols and cellular imaging. Anal. Chim. Acta 2017, 964, 150–160. [Google Scholar] [CrossRef]
- Ye, Q.; Yan, F.; Luo, Y.; Wang, Y.; Zhou, X.; Chen, L. Formation of N, S-codoped fluorescent carbon dots from biomass and their application for the selective detection of mercury and iron ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 854–862. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, W.; Song, W.; Liu, J.; Ren, C.; Wu, J.; Liu, D.; Chen, H. Red Emission B, N, S-co-Doped Carbon Dots for Colorimetric and Fluorescent Dual Mode Detection of Fe3+ Ions in Complex Biological Fluids and Living Cells. ACS Appl. Mater. Interfaces 2017, 9, 12663–12672. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, Z. Simple and Green Synthesis of Boron-, Sulfur-, and Nitrogen-Co-Doped Carbon Dots as Fluorescent Probe for Selective and Sensitive Detection of Sunset Yellow. Nano 2017, 12, 1750123. [Google Scholar] [CrossRef]
- Das, R.K.; Mohapatra, S. Highly luminescent, heteroatom-doped carbon quantum dots for ultrasensitive sensing of glucosamine and targeted imaging of liver cancer cells. J. Mater. Chem. B 2017, 5, 2190–2197. [Google Scholar] [CrossRef]
- Yuan, C.; Zhao, Z.; Sun, X.; Hou, L.; Wang, Z.; Zhang, Y.; Chen, Z. Foxtail millet-derived highly fluorescent multi-heteroatoms doped carbon quantum dots towards fluorescent ink and smart nanosensor for selective ion detection. New J. Chem. 2018, 42, 7326–7331. [Google Scholar]
- Khan, W.U.; Wang, D.; Wang, Y. Highly Green Emissive Nitrogen-Doped Carbon Dots with Excellent Thermal Stability for Bioimaging and Solid-State LED. Inorg. Chem. 2018, 57, 15229–15239. [Google Scholar] [CrossRef]
- Hu, C.; Li, M.; Qiu, J.; Sun, Y.-P. Design and fabrication of carbon dots for energy conversion and storage. Chem. Soc. Rev. 2019. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Ma, D.-K.; Zhang, Y.-G.; Chen, W.; Huang, S.-M. N-doped carbon quantum dots for TiO2-based photocatalysts and dye-sensitized solar cells. Nano Energy 2013, 2, 545–552. [Google Scholar] [CrossRef]
- Wang, H.; Sun, P.; Cong, S.; Wu, J.; Gao, L.; Wang, Y.; Dai, X.; Yi, Q.; Zou, G. Nitrogen-doped carbon dots for “green” quantum dot solar cells. Nanoscale Res. Lett. 2016, 11, 27. [Google Scholar] [CrossRef]
- Mariotti, D.; Padmanaban, D.B.; Rocks, C.; Svrcek, V.; Maguire, P.; Carolan, D. Environmentally friendly nitrogen-doped carbon quantum dots for next generation solar cells. Sustain. Energy Fuels 2017, 1, 1611–1619. [Google Scholar]
- Zhao, Y.; Duan, J.; He, B.; Jiao, Z.; Tang, Q. Improved charge extraction with N-doped carbon quantum dots in dye-sensitized solar cells. Electrochim. Acta 2018, 282, 255–262. [Google Scholar] [CrossRef]
- Yang, Q.; Duan, J.; Yang, W.; Li, X.; Mo, J.; Yang, P.; Tang, Q. Nitrogen-doped carbon quantum dots from biomass via simple one-pot method and exploration of their application. Appl. Surf. Sci. 2018, 434, 1079–1085. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, L.; Ji, G.; Wang, C.; Gu, H.; Luo, Q.; Chen, Q.; Chen, L.; Yang, Y.; Ma, C.-Q.; et al. Synthesis of N,S-Doped Carbon Quantum Dots for Use in Organic Solar Cells as the ZnO Modifier To Eliminate the Light-Soaking Effect. ACS Appl. Mater. Interfaces 2019, 11, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Bandi, R.; Devulapalli, N.P.; Dadigala, R.; Gangapuram, B.R.; Guttena, V. Facile Conversion of Toxic Cigarette Butts to N,S-Codoped Carbon Dots and Their Application in Fluorescent Film, Security Ink, Bioimaging, Sensing and Logic Gate Operation. ACS Omega 2018, 3, 13454–13466. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, L.; Li, Y.; Deng, R.; Zhang, H. Highly fluorescent nitrogen-doped carbon dots with excellent thermal and photo stability applied as invisible ink for loading important information and anti-counterfeiting. Nanoscale 2017, 9, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Ao, K.; Lv, P.; Dong, J.; Wang, D.; Cai, Y.; Wei, Q.; Xu, Y. Fluorescent Nitrogen-Doped Carbon Dots via Single-Step Synthesis Applied as Fluorescent Probe for the Detection of Fe3+ Ions and Anti-Counterfeiting Inks. Nano 2018, 13, 1850097. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2013, 55, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Weng, Y.; Zheng, L.; Yao, B.; Weng, W.; Lin, X. Nitrogen-doped carbon quantum dots as fluorescent probe for “off-on” detection of mercury ions, L-cysteine and iodide ions. J. Colloid Interface Sci. 2017, 506, 373–378. [Google Scholar] [CrossRef]
- Huang, H.; Lv, J.-J.; Zhou, D.-L.; Bao, N.; Xu, Y.; Wang, A.-J.; Feng, J.-J. One-pot green synthesis of nitrogen-doped carbon nanoparticles as fluorescent probes for mercury ions. RSC Adv. 2013, 3, 21691. [Google Scholar] [CrossRef]
- Xu, Q.; Li, B.; Ye, Y.; Cai, W.; Li, W.; Yang, C.; Chen, Y.; Xu, M.; Li, N.; Zheng, X.; et al. Synthesis, mechanical investigation, and application of nitrogen and phosphorus co-doped carbon dots with a high photoluminescent quantum yield. Nano Res. 2018, 11, 3691–3701. [Google Scholar] [CrossRef]
- Chandra, S.; Chowdhuri, A.R.; Mahto, T.K.; Laha, D.; Sahu, S.K. Sulphur and nitrogen doped carbon dots: A facile synthetic strategy for multicolour bioimaging, tiopronin sensing, and Hg2+ ion detection. Nano-Struct. Nano-Objects 2017, 12, 10–18. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, S.H.; Feng, L. Highly luminescent N, S- Co-doped carbon dots and their direct use as mercury(II) sensor. Anal. Chim. Acta 2015, 890, 134–142. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, V.; Yadav, P.K.; Chandra, S.; Bano, D.; Kumar, V.; Koch, B.; Talat, M.; Hasan, S.H. Bright-blue-emission nitrogen and phosphorus-doped carbon quantum dots as a promising nanoprobe for detection of Cr(vi) and ascorbic acid in pure aqueous solution and in living cells. New J. Chem. 2018, 42, 12990–12997. [Google Scholar] [CrossRef]
- Erbas-Cakmak, S.; Kolemen, S.; Sedgwick, A.C.; Gunnlaugsson, T.; James, T.D.; Yoon, J.; Akkaya, E.U. Molecular logic gates: The past, present and future. Chem. Soc. Rev. 2018, 47, 2228–2248. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Li, X.; Yang, H.; Chen, X. Nitrogen-doped carbon dots rapid and selective detection of mercury ion and biothiol and construction of an IMPLICATION logic gate. Talanta 2019, 194, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ma, Q.; Zhang, S.; Liu, H.; Guo, Y.; Feng, F. S,N-Co-doped carbon nanoparticles with high quantum yield for metal ion detection, IMP logic gates and bioimaging applications. New J. Chem. 2018, 42, 20180–20189. [Google Scholar] [CrossRef]
- Sharma, V.; Kaur, N.; Tiwari, P.; Saini, A.K.; Mobin, S.M. Multifunctional fluorescent “Off-On-Off” nanosensor for Au3+ and S2− employing N-S co-doped carbon–dots. Carbon 2018, 139, 393–403. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.C.; Huang, H.; Wang, A.J.; Chen, J.R.; Feng, J.J. Facile synthesis of N, S-codoped fluorescent carbon nanodots for fluorescent resonance energy transfer recognition of methotrexate with high sensitivity and selectivity. Biosens. Bioelectron. 2015, 64, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, J.; Zhao, Y.; Bian, M.; Hou, C.; Huo, D.; Zou, S. Simple and sensitive fluorescence sensor for methotrexate detection based on the inner filter effect of N, S co-doped carbon quantum dots. Anal. Chim. Acta 2018, 1047, 179–187. [Google Scholar]
- Han, Z.; Zhang, H.; He, L.; Pan, S.; Liu, H.; Hu, X. One-pot hydrothermal synthesis of nitrogen and sulfur co-doped carbon dots and their application for sensitive detection of curcumin and temperature. Microchem. J. 2019, 146, 300–308. [Google Scholar] [CrossRef]
- Wu, B.; Liu, X.; Shi, X.; Han, W.; Wang, C.; Jiang, L. Highly photoluminescent and temperature-sensitive P, N, B-co-doped carbon quantum dots and their highly sensitive recognition for curcumin. RSC Adv. 2019, 9, 8340–8349. [Google Scholar] [CrossRef]
- Li, H.; Sun, C.; Vijayaraghavan, R.; Zhou, F.; Zhang, X.; MacFarlane, D.R. Long lifetime photoluminescence in N, S co-doped carbon quantum dots from an ionic liquid and their applications in ultrasensitive detection of pesticides. Carbon 2016, 104, 33–39. [Google Scholar] [CrossRef]
- Zuo, P.; Liu, J.; Guo, H.; Wang, C.; Liu, H.; Zhang, Z.; Liu, Q. Multifunctional N,S co-doped carbon dots for sensitive probing of temperature, ferric ion, and methotrexate. Anal. Bioanal. Chem. 2019, 411, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Guo, F.; Han, M.; Yuan, S.; Guan, W.; Li, H.; Huang, H.; Liu, Y.; Kang, Z. N,S co-doped carbon dots as a stable bio-imaging probe for detection of intracellular temperature and tetracycline. J. Mater. Chem. B 2017, 5, 3293–3299. [Google Scholar] [CrossRef]




















| Synthesis Method | Precursors | Reaction Conditions | Color | QY (%) | Applications | Reference |
|---|---|---|---|---|---|---|
| Boron (B)-Doped CQDs | ||||||
| Solvothermal | Hydroquinone and boron tribromide | 2 h at 200 °C | Blue | 14.8 | Detection of hydrogen peroxide (H2O2) and glucose (LOD of 8 μM) | [61] |
| Microwave | Citric acid, urea, and boric acid | 700 W for 4 min | Green | 15 | - | [62] |
| Hydrothermal | Ascorbic acid and boric acid/sodium borohydride/sodium borate/borax | 7 h at 200 °C | - | 2.1–5.4 | Detection of iron (Fe3+) (LOD of 3.1 nM) ions and ascorbic acid (LOD of 30 nM) | [63] |
| Hydrothermal | Phenylboronic acid | 10 h at 200 °C | - | 12 | Detection of potassium sorbate (LOD of 6.1 nM), and vitamin B12 (LOD of 8 nM) in mineral water, vinegar, bread, vitamin drink, and VB12 tablets. | [64] |
| Fluorine (F)-Doped CQDs | ||||||
| Solvothermal | Citric acid and 4,5-difluorobenzene-1,2-diamine | 8 h at 180 °C | Yellow | 31 | Detection of intracellular Ag+ and cell imaging (HEK 293 and B16F10 cell lines) | [65] |
| Solvothermal | Tetrafluoroterephthalic acid (TFTA) and 1.8K branched polyethyleneimine (1.8K b-PEI) | 6 h at 180° C | Green | - | Gene delivery | [66] |
| Hydrothermal | PEI 600Da and 2,2,3,3,4,4-hexafluoro-1,5-pentanediol diglycidyl ether | 12 h at 180 °C | 5.6 | Gene delivery and cell imaging (HeLa and 7702 cell lines) | [67] | |
| Nitrogen (N)-Doped CQDs | ||||||
| Hydrothermal | Grass | 3 h at 180 °C | Blue | 2.5–6.2 | Label-Free Detection of Cu (II) ions (LOD of 1 nM) | [68] |
| Hydrothermal | Glucose and urea | 1 h at 150 °C | Blue | 0.7 | White light emission | [69] |
| Microwave | 600 W for 7 min | 1 | ||||
| Hydrothermal | Folic acid | 2 h at 150 °C | Blue | 23 | Cell imaging (U87 cell line) | [70] |
| Pyrolysis | Glutamic acid | 5 min at 200 °C | Blue | 28 | Detection of anti-bacterial drug—amoxicillin | [71] |
| Solvothermal | Carbon tertrachloride and diamines—e.g., 1,2-ethylenenediamine, 1,3-propanediamine, or 1,4-butanediamine at different ratios of 2:1, 1:1, 1:2, and 1:3 | 0.5, 1 and 2 h at 200 °C | Blue | 9.8–36.3 | Detection of Ag+, Fe3+, and hydrogen peroxide (H2O2) | [72] |
| Hydrothermal | Dried Monkey grass | 6 h at 180° C | Blue | - | Detection of iodide (I−) ions (LOD of 3.7 μM) | [73] |
| Hydrothermal | Lactose | 3 h at 180 °C | Blue | 10.75 | Detection of pyridine (LOD of 0.03 mM) | [74] |
| Hydrothermal | Citric acid and ethylenediamine | 5 h at 220 °C | Blue | - | Detection of Fe3+ (LOD of 79 nM) ions | [75] |
| Hydrothermal | Tetraphenylporphyrin or its transition metal Pd(II) or Pt(II) complex and ethylenediamine | 20 h at 250 °C | Blue | 10.1, 17.8, and 15.2 | Detection of Fe3+ (LOD of 3.7 μM) ions in aqueous solution and cells, and cell imaging (HeLa cell line) | [76] |
| Hydrothermal | L-histidine | 4 h at 200 °C | Blue | 27 | Detection of Fe3+ (LOD of 1 μM/L) ions | [77] |
| Pyrolysis | Polyvinyl pyrrolidone (PVP, K-30) | 3 h at 400 °C | Blue | 19.6 | Multicolor cell imaging (HeLa cell line) | [78] |
| Hydrothermal | Glycolic-/malic-/citric-acid and urea | 1 h at 250 °C | Blue, cyan, and green | - | Cell imaging (human osteogenesis sarcoma MG-63 cells) | [79] |
| Hydrothermal | 1,2,4-triaminobenzene | 12 h at 120 °C | Yellow | 32.5 | Bifunctional detection of Ag+ (LOD of 0.20 μM) ions and cysteine (Cys) (LOD of 0.25 μM), and cell imaging (MCF-7 cell line) | [80] |
| Hydrothermal | Polyacrylamide | 24 h at 260 °C | - | - | Detection of glucose (LOD of 0.25 mM) in serum samples | [81] |
| Hydrothermal pyrolysis | Tribute chrysanthemum flowers | 24 h at 180 °C | Blue | 17.3 | Label-free detection of Fe3+ ions (LOD of 0.001 M) and hydrazine | [82] |
| Hydrothermal | Ginkgo leaf | 10 h at 200 °C | Blue | 22.8 | Label-free detection of salazosulfapyridine (LOD of 40 nmol/L) in mouse plasma | [83] |
| Hydrothermal | m-aminobenzoic acid | 12 h at 180 °C | Blue | 30.7 | Detection of Fe3+ ions (LOD of 0.05 μM) and pH | [84] |
| Hydrothermal | Microcrystalline cellulose and ethylenediamine | 11 h at 230 °C | Blue | 51 | Detection of Fe3+ ions (LOD of 0.21 nM) in an acidic environment | [85] |
| Solid-phase thermal treatment | Citric acid and dicyandiamide | 1.5 h at 170 °C | Yellowish-green | 73.2 | Label-free probe for detection of Fe3+ (LOD of 50 nM/L) and fluorine (LOD of 75 nM/L) ions | [86] |
| Hydrothermal | Citric acid and ethylenediamine | 150, 200, 250 and 300 °C for 5 h | Blue | 80 | Multicolor patterning for anti-counterfeit applications, detection of Fe3+ ions (LOD of 1ppm) and cell imaging | [87] |
| Microreactor with foamy copper having different poriness values | Citric acid and ethylenediamine | 8 min at 150–230 °C | Blue | 84.1 | Detection of Hg2+ ions (LOD of 2.104 nM) | [88] |
| Thermal pyrolysis | Citric acid and diethylenetriamine | 0.5 h at 170 °C | Blue | 88.6 | Detection of chromium (VI) ions and ascorbic acid | [89] |
| Microwave | Carboxylic acids including 1,2,3,4-butanetetracarboxylic acid/acetic acid/citric acid/malonic acid/oxalic acid/succinic acid, and tris/urea/ethylenediaminetetraacetic acid | 5−45 min at 200−230 °C | Blue | 90 | - | [90] |
| Phosphorus (P)-Doped CQDs | ||||||
| Solvent-thermal reaction | Hydroquinone and phosphorous tribromide | 1, 3, 5 and 9 h at 200 °C | Blue | 25.1 | Cell imaging/biolabeling (HeLa cell line) | [91] |
| Hydrothermal | Sucrose and phosphoric acid | 5 h at 200 °C | Blue | 21.8 | Detection of explosive—2,4,6-trinitrophenol (LOD of 16.9 nM) | [92] |
| Thermal coupling | Citric acid and Na-salt of glycine, l-valine and l-isolucine in the presence of sodium dihydrogen phosphate | 2 h at 200 °C | Green | 15.2, 11 and 19.7 | Cell imaging (HeLa cell line) | [93] |
| Hydrothermal | Concentrated phosphoric acid and triethylphosphonoacetate | 12 h at 200 °C | Blue or orange yellow | - | - | [94] |
| Simple mixing | L-threonine and phosphorus pentoxide | - | Blue | 1.3 | Detection of Au3+ (LOD of 5.86 μM), and L-methionine (LOD of 80 μM/L) | [95] |
| Hydrothermal | Sodium citrate and phytic acid | 4 h at 160, 200 or 240 °C | Blue | 3.5 | Detection of Cu (II) ions (LOD of 1 nM) | [96] |
| Microwave | Ethylenediamine and phytic acid | 700 W for 10 min | Green | 21.65 | Cell imaging/biolabeling (L929 cell line) | [97] |
| Solvothermal | Lactose and phosphoric acid | 20–30 min at 80–90 °C | Yellow | 62 | Detection of Al3+ (LOD of 4 nM) and Zn2+ ions (LOD of 100 nM) | [98] |
| S-Doped CQDs | ||||||
| Chemical process | Thiomalic acid and sulfuric acid | 4 h at 90 °C | Blue | 11.8 | Solar cells, cell imaging (microbial strain of E. coli), and gene delivery | [99] |
| Carbonization | Waste frying oil and sulfuric acid | 5 min at 100 °C | Blue | 3.66 | Cell imaging (HeLa cell line) | [100] |
| Hydrothermal | Sodium citrate and sodium thiosulfate | 6 h at 160, 180, 200, 220, and 240 °C | Blue | 67 | Detection of Fe3+ ions (LOD of 0.1 μM) | [101] |
| Simple heating | Citric acid, urea and sulphur deposits | 15 min at 225 °C | Yellowish green | - | - | [102] |
| Simple heating | Diethylene glycol and 5-sulfosalicylic acid dihydrate | 10 min at 200 °C | Blue | 4 | Visible to near infrared fluorescent probes for hydrogen peroxide (LOD of 0.6 μM) | [103] |
| 30 min at 200 °C | Green | 6.4 | ||||
| Hydrothermal | Poly(sodium4-styrene sulfonate) | 6 h at 200 °C | Blue | 9 | Detection of ammonia | [104] |
| Poly(4-styrene sulfonicacid co-maleic acid) | 6 | |||||
| Microwave-assisted-pyrolysis | Citric acid and cysteamine | 600 W for 3 min | Blue | 49 | Detection of calcium in hard water | [105] |
| Acid carbonization | Sucrose and sulphuric acid | - | Blue | 5.77 | Detection of Fe3+ ions (LOD of 0.56 μM) in highly acidic environment | [106] |
| Hydrothermal | Vitamin B1 (thiamine hydrochloride) and ethylenediamine | 12 h at 200 °C | Blue | 4.4 | Detection of Fe3+ ions (LOD of 177 nM) | [107] |
| B, N Co-Doped CQDs | ||||||
| Hydrothermal | Branched polyethylenimine and 4-formylphenylboronic acid | 8 h at 220 °C | Blue | 15.85 | Detection of Fe3+ ions (LOD of 1.62 μM), and cell imaging (HeLa cell line) | [108] |
| Hydrothermal | Citric acid anhydrous, ethylenediamine, and three different kinds of borate—i.e., sodium tetraborate, boric acid, and manganese borate | 4 h at 160 °C | Blue | 29.01, 51.42, and 68.28 | Detection of Hg2+ ions (LOD of 7.3 nM) and 2,4,6-trinitrophenol (LOD of 0.35 μM), and cell imaging (HUVEC cell line) | [109] |
| Hydrothermal | 2-hydroxyphenylboronic acid and ethylenediamine | 12 h at 180 °C | Yellow-green | 6.59 | Detection of Cr(VI) (LOD of 0.5 μM), anti-counterfeiting and cell imaging (HeLa cell line) | [110] |
| Hydrothermal | 3-aminobenzeneboronic acid and 1,2-ethylenediamine | 7 h at 160 °C | Green | 47 | Determination of α-glucosidase activity and its inhibitors in water samples and living cells | [111] |
| Hydrothermal | 2-aminophenylboronic acid, 3-aminophenylboronic acid monohydrate, or 4-aminophenylboronic acid hydrochloride | 8 h at 160 °C | Blue | ~7 | Detection of p-nitrophenol (LOD of 0.2 μM) | [112,113] |
| Hydrothermal | 3-aminophenylboronic acid | 4 h at 180 °C | 25.9 | Detection of Cr(VI) (LOD of 0.28 μM) and dopamine (LOD of 4.6 μM) | [114] | |
| B, S Co-Doped CQDs | ||||||
| Hydrothermal | Borax and poly(sodium-p-styrenesulfonate) | 8 h at 200 °C | Blue | 25.7 | Detection of diethylstilbestrol (LOD of 0.06 μM) | [115] |
| F, N Co-Doped CQDs | ||||||
| Microwave-assisted carbonation route | Citric acid, urea and sodium fluoride | 750 W for 5 min | Yellow | - | In vitro and in vivo cell imaging | [116] |
| N, P Co-Doped CQDs | ||||||
| Hydrothermal | Trimesic acid, urea, polyethylene diamine branched, and ortho-phosphoric acid | 15 h at 180 °C | Blue | 0.41 | Label free detection of chromium (II) ions (LOD of 0.1 μM) | [117] |
| Low-temperature heating | Sucrose, 1,2-ethylenediamine and phosphoric acid | 50 min at 80 °C | Blue | 12.7 | Detection of hemoglobin (LOD of 0.29 nM) in human urine samples and human blood samples, and also in cell imaging (HepG2 cell line) | [118] |
| Hydrothermal | Pyridoxal 5-phosphate and ethanediamine | 4 h at 180 °C | Blue | 15.4 | Detection of cobalt (Co2+) ions with LOD of 0.053 μM | [119] |
| Microwave-assisted thermolysis | N-phosphonomethyl aminodiacetic acid and ethylenediamine | 700 W for 7 min | Blue | 17.5 | Cell imaging (HeLa cell line) | [120] |
| Hydrothermal | Citric acid and diammonium phosphate | 1 and 4 h at 180 °C | Blue | 59 and 10.58 | (i) Detection of Fe3+ ions in cancer cells and (ii) detection of iodide (LOD of 0.32 μM) and Fe3+ ions (LOD of 72 nM) | [121,122] |
| Acid-base neutralization spontaneous heat | Glucose, 1,2-ethylenediamine, and concentrated phosphoric acid | - | Green | 9.59 | Detection of curcumin (LOD of 58 nmol/L) and cell imaging | [123] |
| Solvothermal | Citric acid, urea, and phosphoric acid in dimethyl formamide solution | 24 h at 180 °C | Greenish yellow | 15 | Detection of Fe3+ ions (LOD of 50 nM) | [124] |
| Hydrothermal | Diethylenetriaminepenta(methylenephosphonic acid) and m-phenylenediamine | 5 h at 200 °C | Green | 32 | Cell imaging (A549 and KB cell line) | [125] |
| Microwave | m-phenylenediamine, ethylenediamine, and ortho-phosphoric acid | 800 W for 40 s | Blue and green | 51 and 38 | Detection of carbendazim (LOD of 0.002 μM) | [127] |
| Hydrothermal | Eleocharisdulcis juice | 5 h at 90, 120, 150 °C | Navy blue, blue, and cyan | 3.3, 11.2 and 8.6 | Fluorescent ink for anti-counterfeiting, and detection of Fe3+ ions (LOD of 0.56 μM) | [128] |
| Hydrothermal | Adenosine-5′-triphosphate | 2 h at 300 °C | Blue | 23.5 | Sensing platform for live cell imaging of reactive oxygen species and reactive nitrogen species, including ClO-, ONOO-, and NO in macrophages | [129] |
| Hydrothermal | Alendronate sodium | 20 h at 180 °C | Blue | 35 | Detection of uranyl ions (LOD of 4.5 nM) in hair and water samples, and also in cell imaging (BT474 cell line) | [130] |
| N, S Co-Doped CQDs | ||||||
| Microwave-assisted pyrolysis | Rice and N-acetyl-L-cysteine (NAC) | 800 W for 30 min | Blue | 2.36 | - | [131] |
| Hydrothermal | Pomegranate juice and L-cysteine | 5 h at 120 °C | Blue | 4.8 | Detection of cephalexin (LOD of 10 μM) | [132] |
| Combustion | Cellulose-based biowaste of willow catkin soaked in N/S aqueous solution containing urea and sulfuric acid | Blue | 13.3 | Detection of Fe3+ ions (LOD of 0.03 μM) and intracellular imaging (HeLa cell line) | [133] | |
| Microwave | Citric acid, urea, and sodium thiosulfate | 700 W for 5 min | - | - | Detection of nitric oxide (LOD of 0.3 μM) in fortified serum solutions | [134] |
| Hydrothermal | Thiourea, urea, and sodium citrate | 6 h at 200 °C | Blue | 16 | Fluorescence quenching studies in bovine hemoglobin | [135] |
| Hydrothermal | Garlic | 10 h at 180 °C | Blue | 13 | Detection of Fe3+ ions (LOD of 0.32 μM) in lake water and tap water, and in cell imaging (RAW264.7 cell line) | [136] |
| Hydrothermal | Garlic | 3 h at 200 °C | Blue | 17.5 | Cell imaging (A549 cell line) and free radical scavenging | [137] |
| Hydrothermal | Garlic and ethylenediamine | 6 h at 200 °C | Blue | 5.1–20.5 | Detection of Fe3+ ions (LOD of 0.2 μM) | [138] |
| Hydrothermal | Citric acid and cystamine dihydrochloride | 6 h at 160 °C | Blue | 39.7 | Detection for Cr(VI) (LOD of 0.86 μM), and multicolor cell imaging (HeLa cell line) | [139] |
| Hydrothermal | α-lipoic acid, sodium hydroxide, and ethylenediamine | 1, 3, 7, 11, 15 and 19 h at 250 °C | Blue | 54.4 | Detection of Fe3+ ions (LOD of 4 μM), and multicolor cell imaging (HeLa cell line) | [140] |
| Hydrothermal | Citric acid and thiamine hydrochloride | 5 h at 160 °C | Blue | 63.8 | Detection of Ag+ ions (LOD of 0.4 μM) and Cys (LOD of 0.35 μM) | [141] |
| Hydrothermal | Citric acid and l-cysteine | 3 h at 200 °C | Violet-Blue | 73 | Cell imaging (HeLa cell line) | [142] |
| Microwave | Citric acid and l-cysteine | 700 W for 40 secs | Blue | 78 ± 10 | Detection of bilirubin (LOD of 0.12 nM) | [143] |
| Solvothermal | Sodium lignosulfonate and p-phenylenediamine | 9 h at 200 °C | Green | 5.7 | Detection of Ag+ ions (LOD of 11.6 μM) and Fe3+ (LOD of 1.7 μM) in real water samples. | [144] |
| Hydrothermal | m-phenylenediamine and concentrated sulfuric acid | 10 h at 200 °C | Yellow | 43 | White light emitting diode (WLED) | [145] |
| Hydrothermal | Kappa carrageenan and urea | 5 h at 210 °C | Green | 69.27 | Detection of acetone (LOD of 72 μM) in human fluids (blood and urine) | [146] |
| Hydrothermal | Allium fistulosum | 3 h at 220 °C | Blue | 10.48 | Multicolor cell imaging in MCF-7 and K562 cell cytoplasm | [147] |
| Hydrothermal | Enteromorpha prolifera (algae genera for green tide) | 3, 6 or 10 h at 180 °C | Blue | 12.3 | Detection of Fe3+ ions (LOD of 0.5 μM) | [148] |
| Hydrothermal | Glutathione | 6 h at 200 °C | Blue | 17.5 | Detection of tetracycline (LOD of 0.04 μM) and temperature probe | [149] |
| Hydrothermal | Casein | 4 h at 180 °C | Blue | 31.8 | Detection of Hg2+ ions (LOD of 6.5 nM) and biothiols such as l-cysteine (LOD of 23.6 nM), homocysteine (LOD of 12.3 nM) and glutathione (LOD of 16.8 nM) | [150] |
| Hydrothermal | Feathers, egg white, egg yolk, and manure from pigeon | 3 h at 300 °C | Blue | 24.87, 17.48, 16.34, and 33.50 | Detection of Hg2+/Fe3+ ions with LOD of 10.3/60.9 nM | [151] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandasamy, G. Recent Advancements in Doped/Co-Doped Carbon Quantum Dots for Multi-Potential Applications. C 2019, 5, 24. https://doi.org/10.3390/c5020024
Kandasamy G. Recent Advancements in Doped/Co-Doped Carbon Quantum Dots for Multi-Potential Applications. C. 2019; 5(2):24. https://doi.org/10.3390/c5020024
Chicago/Turabian StyleKandasamy, Ganeshlenin. 2019. "Recent Advancements in Doped/Co-Doped Carbon Quantum Dots for Multi-Potential Applications" C 5, no. 2: 24. https://doi.org/10.3390/c5020024
APA StyleKandasamy, G. (2019). Recent Advancements in Doped/Co-Doped Carbon Quantum Dots for Multi-Potential Applications. C, 5(2), 24. https://doi.org/10.3390/c5020024