Fluorescence and Physico-Chemical Properties of Hydrogenated Detonation Nanodiamonds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Material Characterization
2.3. Optical Characterization
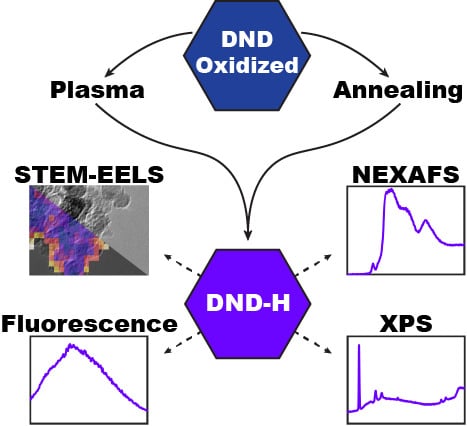
3. Results
3.1. Physico-Chemical Properties
3.1.1. STEM-EELS
3.1.2. NEXAFS, XPS, and FTIR
3.2. Colloidal Properties
3.3. Fluorescence Properties
3.3.1. In-Solution Fluorescence Spectroscopy
3.3.2. Solid-State Fluorescence Spectroscopy
3.3.3. Cryogenic Fluorescence Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DND | Detonation nanodiamond |
| DND-H | Hydrogenated detonation nanodiamond |
| HOPG | Highly-oriented pyrolytic graphite |
| STEM | Scanning transmission electron microscopy |
| EELS | Electron energy loss spectroscopy |
| NEXAFS | Near-edge X-ray absorption fine structure |
| PEY | Partial electron yield |
| XPS | X-ray photoelectron spectroscopy |
| FTIR | Fourier-transform infrared |
| DLS | Dynamic light scattering |
| NV | Nitrogen vacancy |
| HPHT | High-pressure high-temperature |
| DI | Deionized |
References
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The Properties and Applications of Nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Nunn, N.; Torelli, M.; McGuire, G.; Shenderova, O. Nanodiamond: A High Impact Nanomaterial. Curr. Opin. Solid State Mater. Sci. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Lee, D.K.; Kee, T.; Liang, Z.; Hsiou, D.; Miya, D.; Wu, B.; Osawa, E.; Chow, E.K.H.; Sung, E.C.; Kang, M.K.; et al. Clinical Validation of a Nanodiamond-Embedded Thermoplastic Biomaterial. Proc. Natl. Acad. Sci. USA 2017, 114, E9445–E9454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.Q.; Lam, R.; Xu, X.; Chow, E.K.; Kim, H.J.; Ho, D. Multimodal Nanodiamond Drug Delivery Carriers for Selective Targeting, Imaging, and Enhanced Chemotherapeutic Efficacy. Adv. Mater. 2011, 23, 4770–4775. [Google Scholar] [CrossRef] [PubMed]
- Williams, O.A.; Douhéret, O.; Daenen, M.; Haenen, K.; Ōsawa, E.; Takahashi, M. Enhanced Diamond Nucleation on Monodispersed Nanocrystalline Diamond. Chem. Phys. Lett. 2007, 445, 255–258. [Google Scholar] [CrossRef]
- Berman, D.; Narayanan, B.; Cherukara, M.J.; Sankaranarayanan, S.K.R.S.; Erdemir, A.; Zinovev, A.; Sumant, A.V. Operando Tribochemical Formation of Onion-like-Carbon Leads to Macroscale Superlubricity. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pakes, C.I.; Garrido, J.A.; Kawarada, H. Diamond Surface Conductivity: Properties, Devices, and Sensors. MRS Bull. 2014, 39, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Kondo, T.; Neitzel, I.; Mochalin, V.N.; Urai, J.; Yuasa, M.; Gogotsi, Y. Electrical Conductivity of Thermally Hydrogenated Nanodiamond Powders. J. Appl. Phys. 2013, 113, 214307. [Google Scholar] [CrossRef]
- Petit, T.; Puskar, L.; Dolenko, T.; Choudhury, S.; Ritter, E.; Burikov, S.; Laptinskiy, K.; Brzustowski, Q.; Schade, U.; Yuzawa, H.; et al. Unusual Water Hydrogen Bond Network around Hydrogenated Nanodiamonds. J. Phys. Chem. C 2017, 121, 5185–5194. [Google Scholar] [CrossRef] [Green Version]
- Petit, T.; Arnault, J.C.; Girard, H.; Grall, R.; Chevillard, S.; Delic, J. Use of Nanodiamonds for Generating Free Radicals for Therapeutic Purposes under Radiation. U.S. Patent US10,391,172B2, 27 August 2019. [Google Scholar]
- Kurzyp, M.; Girard, H.A.; Cheref, Y.; Brun, E.; Sicard-Roselli, C.; Saada, S.; Arnault, J.C. Hydroxyl Radical Production Induced by Plasma Hydrogenated Nanodiamonds under X-Ray Irradiation. Chem. Commun. 2017, 53, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Grall, R.; Girard, H.; Saad, L.; Petit, T.; Gesset, C.; Combis-Schlumberger, M.; Paget, V.; Delic, J.; Arnault, J.C.; Chevillard, S. Impairing the Radioresistance of Cancer Cells by Hydrogenated Nanodiamonds. Biomaterials 2015, 61, 290–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reineck, P.; Lau, D.W.; Wilson, E.R.; Fox, K.; Field, M.R.; Deeleepojananan, C.; Mochalin, V.N.; Gibson, B.C. Effect of Surface Chemistry on the Fluorescence of Detonation Nanodiamonds. ACS Nano 2017, 11, 10924–10934. [Google Scholar] [CrossRef] [PubMed]
- Mochalin, V.N.; Osswald, S.; Portet, C.; Yushin, G.; Hobson, C.; Havel, M.; Gogotsi, Y. High Temperature Functionalization and Surface Modification of Nanodiamond Powders. MRS Online Proc. Libr. Arch. 2007, 1039. [Google Scholar] [CrossRef]
- Girard, H.; Arnault, J.; Perruchas, S.; Saada, S.; Gacoin, T.; Boilot, J.P.; Bergonzo, P. Hydrogenation of Nanodiamonds Using MPCVD: A New Route toward Organic Functionalization. Diam. Relat. Mater. 2010, 19, 1117–1123. [Google Scholar] [CrossRef]
- Arnault, J.; Girard, H. Hydrogenated Nanodiamonds: Synthesis and Surface Properties. Curr. Opin. Solid State Mater. Sci. 2017, 21, 10–16. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, C.; Liu, C. Surface Modification and Intrinsic Green Fluorescence Emission of a Detonation Nanodiamond. J. Mater. Chem. C 2013, 1, 6630–6636. [Google Scholar] [CrossRef]
- Reineck, P.; Lau, D.W.; Wilson, E.R.; Nunn, N.; Shenderova, O.A.; Gibson, B.C. Visible to Near-IR Fluorescence from Single-Digit Detonation Nanodiamonds: Excitation Wavelength and pH Dependence. Sci. Rep. 2018, 8, 2478. [Google Scholar] [CrossRef] [Green Version]
- Vervald, A.M.; Burikov, S.A.; Shenderova, O.A.; Nunn, N.; Podkopaev, D.O.; Vlasov, I.I.; Dolenko, T.A. Relationship Between Fluorescent and Vibronic Properties of Detonation Nanodiamonds and Strength of Hydrogen Bonds in Suspensions. J. Phys. Chem. C 2016, 120, 19375–19383. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Mochalin, V.N. Biomedical Applications of Nanodiamond (Review). Nanotechnology 2017, 28, 252001. [Google Scholar] [CrossRef]
- Bradac, C.; Gaebel, T.; Naidoo, N.; Sellars, M.; Twamley, J.; Brown, L.; Barnard, A.; Plakhotnik, T.; Zvyagin, A.; Rabeau, J. Observation and Control of Blinking Nitrogen-Vacancy Centres in Discrete Nanodiamonds. Nat. Nanotechnol. 2010, 5, 345. [Google Scholar] [CrossRef] [Green Version]
- Reineck, P.; Capelli, M.; Lau, D.; Jeske, J.; Field, M.; Ohshima, T.; Greentree, A.; Gibson, B. Bright and Photostable Nitrogen-Vacancy Fluorescence from Unprocessed Detonation Nanodiamond. Nanoscale 2017, 9, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Terada, D.; Segawa, T.F.; Shames, A.I.; Onoda, S.; Ohshima, T.; Ōsawa, E.; Igarashi, R.; Shirakawa, M. Monodisperse Five-Nanometer-Sized Detonation Nanodiamonds Enriched in Nitrogen-Vacancy Centers. ACS Nano 2019, 13, 6461–6468. [Google Scholar] [CrossRef] [PubMed]
- Osswald, S.; Yushin, G.; Mochalin, V.; Kucheyev, S.O.; Gogotsi, Y. Control of sp2/sp3 Carbon Ratio and Surface Chemistry of Nanodiamond Powders by Selective Oxidation in Air. J. Am. Chem. Soc. 2006, 128, 11635–11642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Q.; Fujiwara, M.; Okano, M.; Takeuchi, S. Observation of 1.2-GHz Linewidth of Zero-Phonon-Line in Photoluminescence Spectra of Nitrogen Vacancy Centers in Nanodiamonds Using a Fabry-Perot Interferometer. Opt. Express OE 2013, 21, 29679–29686. [Google Scholar] [CrossRef] [Green Version]
- Manson, N.B.; Hedges, M.; Barson, M.S.J.; Ahlefeldt, R.; Doherty, M.W.; Abe, H.; Ohshima, T.; Sellars, M.J. NV−–N+ Pair Centre in 1b Diamond. New J. Phys. 2018, 20, 113037. [Google Scholar] [CrossRef]
- Cowie, B.C.C.; Tadich, A.; Thomsen, L. The Current Performance of the Wide Range (90–2500 eV) Soft X-ray Beamline at the Australian Synchrotron. AIP Conf. Proc. 2010, 1234, 307–310. [Google Scholar] [CrossRef]
- Watts, B.; Thomsen, L.; Dastoor, P.C. Methods in Carbon K-Edge NEXAFS: Experiment and Analysis. J. Electron Spectrosc. Relat. Phenom. 2006, 151, 105–120. [Google Scholar] [CrossRef]
- Gann, E.; McNeill, C.R.; Tadich, A.; Cowie, B.C.C.; Thomsen, L. Quick AS NEXAFS Tool (QANT): A Program for NEXAFS Loading and Analysis Developed at the Australian Synchrotron. J. Synchrotron Radiat. 2016, 23, 374–380. [Google Scholar] [CrossRef]
- Reineck, P.; Trindade, L.F.; Havlik, J.; Stursa, J.; Heffernan, A.; Elbourne, A.; Orth, A.; Capelli, M.; Cigler, P.; Simpson, D.A.; et al. Not All Fluorescent Nanodiamonds Are Created Equal: A Comparative Study. Part. Part. Syst. Charact. 2019, 36, 1900009. [Google Scholar] [CrossRef]
- Xu, N.S.; Chen, J.; Deng, S.Z. Effect of Heat Treatment on the Properties of Nano-Diamond under Oxygen and Argon Ambient. Diam. Relat. Mater. 2002, 11, 249–256. [Google Scholar] [CrossRef]
- Gruen, D.M.; Krauss, A.R.; Zuiker, C.D.; Csencsits, R.; Terminello, L.J.; Carlisle, J.A.; Jimenez, I.; Sutherland, D.G.J.; Shuh, D.K.; Tong, W.; et al. Characterization of Nanocrystalline Diamond Films by Core-level Photoabsorption. Appl. Phys. Lett. 1996, 68, 1640–1642. [Google Scholar] [CrossRef]
- Morar, J.F.; Himpsel, F.J.; Hollinger, G.; Hughes, G.; Jordan, J.L. Observation of a C1s Core Exciton in Diamond. Phys. Rev. Lett. 1985, 54, 1960–1963. [Google Scholar] [CrossRef] [PubMed]
- Shpilman, Z.; Gouzman, I.; Minton, T.K.; Shen, L.; Stacey, A.; Orwa, J.; Prawer, S.; Cowie, B.C.C.; Hoffman, A. A near Edge X-Ray Absorption Fine Structure Study of Oxidized Single Crystal and Polycrystalline Diamond Surfaces. Diam. Relat. Mater. 2014, 45, 20–27. [Google Scholar] [CrossRef]
- Stacey, A.; Cowie, B.C.C.; Orwa, J.; Prawer, S.; Hoffman, A. Diamond C1s Core-Level Excitons: Surface Sensitivity. Phys. Rev. B 2010, 82, 125427. [Google Scholar] [CrossRef]
- Stöhr, J. NEXAFS Spectroscopy; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Fallon, P.J.; Veerasamy, V.S.; Davis, C.A.; Robertson, J.; Amaratunga, G.A.J.; Milne, W.I.; Koskinen, J. Properties of Filtered-Ion-Beam-Deposited Diamondlike Carbon as a Function of Ion Energy. Phys. Rev. B 1993, 48, 4777–4782. [Google Scholar] [CrossRef] [PubMed]
- Bobrov, K.; Comtet, G.; Dujardin, G.; Hellner, L.; Bergonzo, P.; Mer, C. Surface Electronic States of the Partially Hydrogenated Diamond C(100)-(2x1):H Surface. Phys. Rev. B 2001, 63, 165421. [Google Scholar] [CrossRef]
- Jaouen, M.; Tourillon, G.; Delafond, J.; Junqua, N.; Hug, G. A NEXAFS Characterization of Ion-Beam-Assisted Carbon-Sputtered Thin Films. Diam. Relat. Mater. 1995, 4, 200–206. [Google Scholar] [CrossRef]
- Lenardi, C.; Baker, M.A.; Briois, V.; Coccia Lecis, G.; Piseri, P.; Gissler, W. Near-Edge X-Ray Absorption Fine Structure Study of Carbon Nitride Films. Surf. Coat. Technol. 2000, 125, 317–321. [Google Scholar] [CrossRef]
- Laikhtman, A.; Hoffman, A. Interaction of Thermally Activated and Molecular Oxygen with Hydrogenated Polycrystalline Diamond Surfaces Studied by Synchrotron Radiation Techniques. Surf. Sci. 2003, 522, L1–L8. [Google Scholar] [CrossRef]
- Hoffman, A.; Comtet, G.; Hellner, L.; Dujardin, G.; Petravic, M. Surface Near-Edge x-Ray Adsorption Fine Structure of Hydrogenated Diamond Films and Di(100) Surfaces Studied by H+ and H- Ion Desorption. Appl. Phys. Lett. 1998, 73, 1152–1154. [Google Scholar] [CrossRef]
- Parry, D.E. Atomic Calculation of Photoionization Cross-Sections and Asymmetry Parameters J.-J. YEH, Published by Gordon and Breach, Langhorne PA, 1993 ISBN 2-88124-585-4. Rapid Commun. Mass Spectrom. 1994, 8, 579. [Google Scholar] [CrossRef]
- Stehlik, S.; Glatzel, T.; Pichot, V.; Pawlak, R.; Meyer, E.; Spitzer, D.; Rezek, B. Water Interaction with Hydrogenated and Oxidized Detonation Nanodiamonds—Microscopic and Spectroscopic Analyses. Diam. Relat. Mater. 2016, 63, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.; Jiang, T.; Xu, K.; Li, S. FTIR Study of the Adsorption of Water on Ultradispersed Diamond Powder Surface. Appl. Surf. Sci. 1998, 133, 231–238. [Google Scholar] [CrossRef]
- Shenderova, O.A.; Vlasov, I.I.; Turner, S.; Van Tendeloo, G.; Orlinskii, S.B.; Shiryaev, A.A.; Khomich, A.A.; Sulyanov, S.N.; Jelezko, F.; Wrachtrup, J. Nitrogen Control in Nanodiamond Produced by Detonation Shock-Wave-Assisted Synthesis. J. Phys. Chem. C 2011, 115, 14014–14024. [Google Scholar] [CrossRef]
- Arnault, J. X-Ray Photoemission Spectroscopy Applied to Nanodiamonds: From Surface Chemistry to in Situ Reactivity. Diam. Relat. Mater. 2018. [Google Scholar] [CrossRef]
- Chung, P.H.; Perevedentseva, E.; Cheng, C.L. The Particle Size-Dependent Photoluminescence of Nanodiamonds. Surf. Sci. 2007, 601, 3866–3870. [Google Scholar] [CrossRef]
- Cheng, C.L.; Chen, C.F.; Shaio, W.C.; Tsai, D.S.; Chen, K.H. The CH Stretching Features on Diamonds of Different Origins. Diam. Relat. Mater. 2005, 14, 1455–1462. [Google Scholar] [CrossRef]
- Ando, T.; Inoue, S.; Ishii, M.; Kamo, M.; Sato, Y.; Yamada, O.; Nakano, T. Fourier-Transform Infrared Photoacoustic Studies of Hydrogenated Diamond Surfaces. J. Chem. Soc. Faraday Trans. 1993, 89, 749–751. [Google Scholar] [CrossRef]
- Williams, O.A.; Hees, J.; Dieker, C.; Jäger, W.; Kirste, L.; Nebel, C.E. Size-Dependent Reactivity of Diamond Nanoparticles. ACS Nano 2010, 4, 4824–4830. [Google Scholar] [CrossRef]
- Petit, T.; Puskar, L. FTIR Spectroscopy of Nanodiamonds: Methods and Interpretation. Diam. Relat. Mater. 2018, 89, 52–66. [Google Scholar] [CrossRef]
- Ozawa, M.; Inaguma, M.; Takahashi, M.; Kataoka, F.; Krüger, A.; Ōsawa, E. Preparation and Behavior of Brownish, Clear Nanodiamond Colloids. Adv. Mater. 2007, 19, 1201–1206. [Google Scholar] [CrossRef]
- Shenderova, O.; Grichko, V.; Hens, S.; Walch, J. Detonation Nanodiamonds as UV Radiation Filter. Diam. Relat. Mater. 2007, 16, 2003–2008. [Google Scholar] [CrossRef]
- Eidelman, E.D.; Siklitsky, V.I.; Sharonova, L.V.; Yagovkina, M.A.; Vul’, A.Y.; Takahashi, M.; Inakuma, M.; Ozawa, M.; Ōsawa, E. A Stable Suspension of Single Ultrananocrystalline Diamond Particles. Diam. Relat. Mater. 2005, 14, 1765–1769. [Google Scholar] [CrossRef]
- Reineck, P.; Francis, A.; Orth, A.; Lau, D.W.M.; Nixon-Luke, R.D.V.; Rastogi, I.D.; Razali, W.A.W.; Cordina, N.M.; Parker, L.M.; Sreenivasan, V.K.A.; et al. Brightness and Photostability of Emerging Red and Near-IR Fluorescent Nanomaterials for Bioimaging. Adv. Opt. Mater. 2016, 4, 1549–1557. [Google Scholar] [CrossRef]
- Chang, S.L.Y.; Barnard, A.S.; Dwyer, C.; Boothroyd, C.B.; Hocking, R.K.; Ōsawa, E.; Nicholls, R.J. Counting Vacancies and Nitrogen-Vacancy Centers in Detonation Nanodiamond. Nanoscale 2016, 8, 10548–10552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaviani, M.; Deák, P.; Aradi, B.; Frauenheim, T.; Chou, J.P.; Gali, A. Proper Surface Termination for Luminescent Near-Surface NV Centers in Diamond. Nano Lett. 2014, 14, 4772–4777. [Google Scholar] [CrossRef] [PubMed]
- Stacey, A.; Dontschuk, N.; Chou, J.P.; Broadway, D.A.; Schenk, A.K.; Sear, M.J.; Tetienne, J.P.; Hoffman, A.; Prawer, S.; Pakes, C.I.; et al. Evidence for Primal Sp2 Defects at the Diamond Surface: Candidates for Electron Trapping and Noise Sources. Adv. Mater. Interfaces 2019, 6, 1801449. [Google Scholar] [CrossRef]
- Stacey, A.; Karle, T.J.; McGuinness, L.P.; Gibson, B.C.; Ganesan, K.; Tomljenovic-Hanic, S.; Greentree, A.D.; Hoffman, A.; Beausoleil, R.G.; Prawer, S. Depletion of Nitrogen-vacancy Color Centers in Diamond via Hydrogen Passivation. Appl. Phys. Lett. 2012, 100, 071902. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Trinchi, A.; Atkin, P.; Cole, I. Tunable Photoluminescence Across the Entire Visible Spectrum from Carbon Dots Excited by White Light. Angew. Chem. Int. Ed. 2015, 54, 2970–2974. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]






| Sample | Aggregate Diameter (nm) | -Potential (mV) | sp-Bonded Carbon (%) |
|---|---|---|---|
| DND-H Anneal | ≈20–200 | 72 ± 7 | 19 |
| DND-H Plasma | ≈20–200 | 53 ± 10 | 19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thalassinos, G.; Stacey, A.; Dontschuk, N.; Murdoch, B.J.; Mayes, E.; Girard, H.A.; Abdullahi, I.M.; Thomsen, L.; Tadich, A.; Arnault, J.-C.; et al. Fluorescence and Physico-Chemical Properties of Hydrogenated Detonation Nanodiamonds. C 2020, 6, 7. https://doi.org/10.3390/c6010007
Thalassinos G, Stacey A, Dontschuk N, Murdoch BJ, Mayes E, Girard HA, Abdullahi IM, Thomsen L, Tadich A, Arnault J-C, et al. Fluorescence and Physico-Chemical Properties of Hydrogenated Detonation Nanodiamonds. C. 2020; 6(1):7. https://doi.org/10.3390/c6010007
Chicago/Turabian StyleThalassinos, Giannis, Alastair Stacey, Nikolai Dontschuk, Billy J. Murdoch, Edwin Mayes, Hugues A. Girard, Ibrahim M. Abdullahi, Lars Thomsen, Anton Tadich, Jean-Charles Arnault, and et al. 2020. "Fluorescence and Physico-Chemical Properties of Hydrogenated Detonation Nanodiamonds" C 6, no. 1: 7. https://doi.org/10.3390/c6010007
APA StyleThalassinos, G., Stacey, A., Dontschuk, N., Murdoch, B. J., Mayes, E., Girard, H. A., Abdullahi, I. M., Thomsen, L., Tadich, A., Arnault, J.-C., Mochalin, V. N., Gibson, B. C., & Reineck, P. (2020). Fluorescence and Physico-Chemical Properties of Hydrogenated Detonation Nanodiamonds. C, 6(1), 7. https://doi.org/10.3390/c6010007