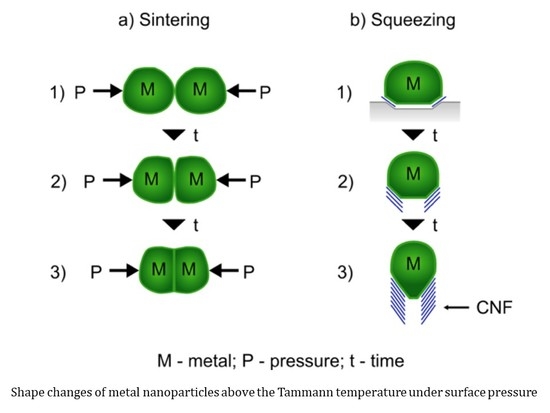
Explaining Bamboo-Like Carbon Fiber Growth Mechanism: Catalyst Shape Adjustments above Tammann Temperature
Abstract
:1. Introduction
2. Growth of Carbon Nanotubes above Catalysts Tammann Temperature (TTa)
3. Alternative Routes and Shapes
4. Opening and Filling Observed in MWCNTs
5. Bamboo-Like Hexagonal Boron Nitride (h-BN) Nanotubes and Thin Films
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jia, Z.; Kou, K.; Qin, M.; Wu, H.; Puleo, F.; Liotta, F. Controllable and large- scale synthesis of carbon Nanostructures: A Review of Bamboo-Like Nanotubes. Catalysts 2017, 7, 256. [Google Scholar] [CrossRef] [Green Version]
- Lobo, L.S. Catalytic carbon formation: Clarifying the alternative kinetic routes and defining a kinetic linearity for sustained growth concept. React. Kinet. Mech. Cat. 2016, 118, 393–414. [Google Scholar] [CrossRef]
- Barrer, R.M. Aspects of gas-metal equilibrium, interstitial solution and diffusion. Discuss. Faraday Soc. 1948, 4, 68–81. [Google Scholar] [CrossRef]
- Barrer, R.M. Diffusion in and through Solids; The University Press, MacMillan: Cambridge, UK, 1941. [Google Scholar]
- Cordero, B.; Gomez, V.; Platero-Prats, A.E.; Revés, M.; Echeverria, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Lobo, L.S.; Carabineiro, S.A.C. Kinetics and mechanism of catalytic carbon gasification. Fuel 2016, 183, 457–469. [Google Scholar] [CrossRef]
- Lee, C.J.; Park, J. Growth model of bamboo-shaped carbon nanotubes by thermal chemical vapor deposition. Appl. Phys. Lett. 2000, 77, 3397–3399. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.; Parker, C.B.; Stoner, B.R.; Glass, J.T. Growth of vertically aligned bamboo-like carbon nanotubes from ammonia/methane precursors using a platinum catalyst. Carbon 2011, 49, 266–274. [Google Scholar] [CrossRef]
- Gonzalez, I.; de Jesus, J.C.; Canizales, E. Bamboo-shaped CNTs generated by methane thermal decomposition using Ni nanoparticles synthesized in water-oil emulsions. Micron 2011, 42, 819–825. [Google Scholar] [CrossRef]
- De Jesus, J.C.; Gonzalez, I.; Garcia, M.; Urbina, C. Preparation of nickel nanoparticles and their catalytic activity in the cracking of methane. J. Vac. Sci. Technol. A 2008, 26, 913–918. [Google Scholar] [CrossRef]
- Zaikovski, V.I.; Chesnokov, V.V.; Buyanov, R.A. The Relationship between the State of Active Species in a Ni/Al2O3 Catalyst and the Mechanism of Growth of Filamentous Carbon. Kinet. Catal. 2001, 42, 890–931. [Google Scholar] [CrossRef]
- McKee, D.W. Metal oxides as catalysts for oxidation of graphite. Carbon 1970, 8, 623–626. [Google Scholar] [CrossRef]
- Sharma, R.; Rez, P.; Brown, M.; Du, G.; Treacy, M.M.J. Dynamic observations of the effect of pressure and temperature conditions on the selective synthesis of carbon nanotubes. Nanotechnology 2007, 18, 125602–125609. [Google Scholar] [CrossRef]
- Hofmann, S.; Sharma, R.; Ducati, C.; Du, G.; Mattevi, C.; Cepek, C.; Cantoro, M.; Pisana, S.; Parvez, A.; Cervantes-Sodi, F.; et al. In situ observations of Catalyst Dynamics during Surface-Bound Carbon Nanotube Nucleation. Nano Lett. 2007, 7, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Chen, D.; Totdal, B.; Holmen, A.H. Effect of support and Reactant on the Yield and Structure of Carbon Growth by Chemical vapor deposition. J. Phys. Chem. B 2005, 109, 6096–6102. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.B.; Ascensio, J.A.; Perez-Alvarez, M.; Yacaman, M.J. Melting behavior of nanometer sized gold isomers. Surf. Sci. 2001, 491, 88–98. [Google Scholar] [CrossRef]
- Saito, Y. Nanoparticles and filled nanocapsules. Carbon 1995, 33, 979–988. [Google Scholar] [CrossRef]
- Li, Y.D.; Chen, J.; Ma, Y.; Zhao, J.; Qin, Y.; Chang, L. Formation of bamboo-like nanocarbon and evidence for the quasi-liquid state of nanosized metal particles at moderate temperatures. Chem. Comm. 1999, 1141–1142. [Google Scholar] [CrossRef]
- Cui, H.; Zhou, O.; Stoner, B.R. Deposition of aligned bamboo-like carbon nanotubes via microwave plasma enhanced chemical vapor deposition. J. Appl. Phys. 2000, 88, 6072–6074. [Google Scholar] [CrossRef]
- He, C.; Zhao, N.; Shi, C.; Du, X.; Li, J. TEM studies of the initial stage growth and morphologies of bamboo-shaped carbon nanotubes synthesized by CVD. J. Alloys Comp. 2007, 433, 79–83. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Ma, Y.; Qin, Y.; Chang, L. Formation of bamboo-shaped carbon filaments and dependence of their morphology on catalyst composition and reaction conditions. Carbon 2001, 39, 1467–1475. [Google Scholar] [CrossRef]
- Jung, M.; Eun, K.Y.; Lee, J.-K.; Baik, Y.-J.; Lee, K.-R.; Park, J.W. Growth of CNTs by CVD. Diamond Rel. Mater. 2001, 10, 1235–1240. [Google Scholar] [CrossRef]
- Katayama, T.; Araki, H.; Yoshino, K. Multiwalled nanotubes with bamboo-like structure and effects of heat treatment. J. Appl. Phys. 2002, 91, 6675–6678. [Google Scholar] [CrossRef]
- Chadderton, L.T.; Chen, Y. A model for the growth of bamboo and skeletal nanotubes: Catalytic capillarity. J. Crystal Growth 2002, 240, 164–169. [Google Scholar] [CrossRef]
- Bartsch, K.; Biedermann, J.; Gemming, T.; Leonhardt, A. On the diffusion-controlled growth of multi-walled CNTs. J. Appl. Phys. 2005, 97, 114301. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, C.E.; Lee, T.J.; Lyu, S.C.; Lee, C.J. Lateral force microscopy of bamboo-shaped multiwalled CNTs. Curr. Appl. Phys. 2006, 6, 141–144. [Google Scholar] [CrossRef]
- Ting, J.-M.; Lin, S.-H. Growth and characteristics of CNTs obtained under different C2H2/H2/NH3 concentrations. Carbon 2007, 45, 1934–1940. [Google Scholar] [CrossRef]
- Lin, M.T.; Tan, J.P.I.; Boothroyd, C.; Loh, K.P.; Tok, E.S.; Foo, Y.-L. Dynamical Observation of Bamboo-like Carbon Nanotube Growth. Nano Lett. 2007, 7, 2234–2238. [Google Scholar] [CrossRef]
- Katar, S.L.; Gonzalez-Berrios, A.; de Jesus, J.; Weiner, B.; Morell, G. Direct deposition of Bamboo-Like Carbon Nanotubes on Copper Substrates by Sulfur-Assisted HFCVD. J. Nanomat. 2008. article 515890 (7 pages). [Google Scholar] [CrossRef] [Green Version]
- Xue, B.; Liu, R.; Huang, W.-Z.; Zheng, Y.-F.; Xu, Z.-D. Growth and characterization of bamboo-like multiwalled carbon nanotubes over Cu/Al2O3 catalyst. J. Mater. Sci. 2009, 44, 4040–4046. [Google Scholar] [CrossRef]
- Wang, C.; Zhan, L.; Wang, Y.-L.; Qiau, W.-M.; Liang, X.; Ling, L.-C. Effect of Sulfur on the growth of carbon nanotubes by detonation-assisted CVD. Appl. Surf. Sci. 2010, 257, 932–936. [Google Scholar] [CrossRef]
- Lin, J.-H.; Chen, C.-S.; Zheng, Z.-Y.; Chang, C.-W.; Chen, H.-W. Sulphate-activated growth of bamboo-like carbon nanotubes over copper catalysts. Nanoscale 2012, 4, 4757–4764. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jia, J.; Kwong, F.; Ng, D.H.L. Synthesis of bamboo-like CNTs on a Cu foil by catalytic CVD from ethanol. Carbon 2012, 50, 2504–2512. [Google Scholar] [CrossRef]
- Keczenovity, E.; Fejes, D.; Reti, B.; Hernadi, K. Growth and characterization of bamboo-like carbon nanotubes synthesized on Fe-Co-Cu catalysts prepared by high-energy ball milling. Phys. Status Solidi B 2013, 250, 2544–2548. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, J.H. Purity-controllable growth of bamboo-like multi-walled CNTs over copper-based catalysts. Catal. Commun. 2013, 34, 41–44. [Google Scholar] [CrossRef]
- Krishna, V.M.; Abilarassu, A.; Somanathan, T.; Gokulakrishnan, N. Effective synthesis of well graphitized high yield bamboo-like MWCNTs on copper loaded α-alumina nanoparticles. Diamond Rel. Mater. 2014, 50, 20–25. [Google Scholar] [CrossRef]
- Velasquez, M.; Batiot-Dupeyrat, C.; Gallego, J.; Santamaria, A. Chemical and morphological characterization of MWCNTs synthesized by C deposition from ethanol-glycerol blend. Diamond Rel. Mater. 2014, 50, 30–48. [Google Scholar] [CrossRef]
- Boi, F.S.; Wang, S.; He, Y. Mapping the transition from catalyst-pool to bamboo-like growth-mechanism in VA free-standing films of CNTs filled with Fe3C: The key role of water. AIP Adv. 2016, 6, 0851012016. [Google Scholar] [CrossRef] [Green Version]
- Sarawast, S.K.; Sinha, B.; Pant, K.K.; Gupta, R.B. Kinetic Study and Modeling of Homogeneous Thermocatalytic Decomposition of Methane over a Ni-Cu-Zn/Al2O3 Catalyst for the Production of Hydrogen and Bamboo-Shaped CNTs. Ind. Eng. Chem. Res. 2016, 55, 11672–11680. [Google Scholar] [CrossRef]
- Wang, Y.; McIntyre, P.C.; Cai, W. Phase Field Model for Morphological Transition in Nanowire Vapor-Liquid-Solid Growth. Crystal Growth Des. 2017, 17, 2211–2227. [Google Scholar] [CrossRef]
- Arnaiz, N.; Martin-Gullon, I.; Font, R.; Gomez-Rico, M.F. Production of bamboo-type carbon nanotubes doped with nitrogen from polyamide pyrolysis gas. J. Anal. Appl. Pyrol. 2018, 130, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Luo, W. Synthesis and Characterization of Bamboo-like MWCNTs By Alcohothermal Process. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 012058. [Google Scholar] [CrossRef] [Green Version]
- Kumi, D.O.; Phaahlamohlaka, T.N.; Dlamini, M.W.; Mangezvo, I.T.; Mhlanga, S.D.; Scurrell, M.S.; Coville, N.J. Effect of titania covering on CNTS as support for the Ru catalyzed selective Co metanation. Appl. Cat. B Environ. 2018, 232, 492–500. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.-F.; Bando, Y. Structures of a hollow filamentary conical helix. Acta Cryst. A 2003, 59, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Merkulov, V.I.; Guillom, M.A.; Lowndes, D.H.; Simpson, M.L.; Voelkl, E. Shaping carbon nanostructures by controlling the synthesis process. Appl. Phys. Lett. 2001, 79, 1178–1180. [Google Scholar] [CrossRef] [Green Version]
- Merkulov, V.I.; Hensley, D.K.; Melechko, A.V.; Guillorn, M.A.; Lowndes, D.H.; Simpson, M.L. Control Mechanisms for the Growth of Isolated Vertically Aligned Carbon Nanofibers. J. Phys. Chem. B 2002, 106, 10570–10577. [Google Scholar] [CrossRef] [Green Version]
- Melechko, A.V. Vertically aligned CNF and related structures: Controlled synthesis and directed assembly. J. Appl. Phys. 2005, 97, 041301. [Google Scholar] [CrossRef]
- Merkulov, I.A.; Yoon, M.; Geohegan, D.B. How the shape of catalyst nanoparticles determines their crystallographic orientation during carbon nanofiber growth. Carbon 2013, 60, 41–45. [Google Scholar] [CrossRef]
- Becker, M.J.; Xia, W.; Tessonier, J.-P.; Blume, R.; Yao, L.; Schlögl, R.; Muhler, M. Optimizing the synthesis of cobalt-based catalysts for the selective growth of multiwalled CNTs under industrially relevant conditions. Carbon 2011, 49, 5253–5264. [Google Scholar] [CrossRef] [Green Version]
- Maurice, J.-L.; Pribat, D.; He, Z.; Patriarche, G.; Cojocaru, C.S. Catalyst faceting during graphene layer crystallization in the course of carbon nanofiber growth. Carbon 2014, 79, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Kovalevski, V.V.; Safronov, A.N. Pyrolysis of Hollow Carbons Melted Catalyst. Carbon 1998, 36, 963–968. [Google Scholar] [CrossRef]
- Bartsch, K.; Leonhardt, A. An approach to the structural diversity of aligned grown multi-walled CNTs on catalyst layers. Carbon 2004, 42, 1731–1736. [Google Scholar] [CrossRef]
- Mohammad, S.N. Systematic investigation of the growth mechanism for conventional, doped and bamboo-shaped nanotubes. Carbon 2014, 75, 133–148. [Google Scholar] [CrossRef]
- Harris, P.J.F. Carbon Nanotube Science: Synthesis, Properties and Applications; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2011. [Google Scholar]
- Wu, C.; Sahajwalla, V. Influence of Melt Carbon and Sulfur on the Wetting of Solid Graphite by Fe-C-S Melts. Metallurg. Mater. Trans. B 1998, 29, 471–477. [Google Scholar] [CrossRef]
- Luo, N.; Jiu, H. Gaseous detonation fabrication of CNTs and CNTs doping with Fe based composites. Fuller. Nanotub. Carb. Nanostruct. 2016, 24, 494–499. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, Z.; Wu, W.; Liu, Z. Detonation chemistry of a CHNO explosive: Catalytic assembly of carbon nanotubes at low pressure and temperature state. Chem. Commun. 2002, 2740–2741. [Google Scholar] [CrossRef]
- Shaikjee, A.; Coville, N.J. The role of the hydrocarbon source on the growth of carbon materials. Carbon 2012, 50, 33376–33398. [Google Scholar] [CrossRef]
- Sun, L.; Banhart, F.; Krasheninnikov, A.V.; Rodrigues-Manzo, J.A.; Terrones, H.; Ajyan, P.M. Carbon nanotubes as high-pressure cylinders and nanoextruders. Science 2006, 312, 1199–1202. [Google Scholar] [CrossRef] [Green Version]
- Amelinckx, S.; Zhang, X.B.; Bernaerts, D.; Zhang, X.F.; Ivanov, V.; Nagy, B.A. Formation Mechanism for Catalytically Grown Helix-Shaped Graphite Nanotubes. Science 1994, 265, 635–639. [Google Scholar] [CrossRef]
- Usman, I.B.; Matsoso, M.; Ranganathan, K.; Naidoo, D.; Coville, N.J.; Wamwangi, D. Magnetic properties of iron containing N-doped MWCNTs. Mater. Chem. Phys. 2018, 209, 280–290. [Google Scholar] [CrossRef]
- Coville, N.J.; Mhlanga, S.D.; Nxumalo, E.N.; Saikjee, A. A review of shaped carbon nanomaterials. S. Afr. J. Sci. 2011, 107, 1–15. [Google Scholar] [CrossRef]
- Han, W.; Bando, Y.; Kurashima, K.; Sato, T. Synthesis of boron nitride nanotubes from carbon nanotubes by a substitution reaction. Appl. Phys. Lett. 1998, 73, 3085–3087. [Google Scholar] [CrossRef]
- Ma, Y.; Bando, Y.; Sato, T. Controlled Synthesis of BN Nanotubes, Nanobamboos and Nanocables. Adv. Mater. 2002, 14, 366–368. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Gu, Y.; Zhao, G.; Qian, Q.; Li, J.; Pan, X.; Zhang, Z. Catalytic growth of bamboo-like boron nitride nanotubes using self-propagation HT synthesized porous precursor. Mater. Lett. 2012, 67, 17–20. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Zhao, G.; Gu, Y.; Zhang, Z. Selective synthesis of boron nitride nanotubes by self-propagation high temperature synthesis and annealing process. J. Solid State Chem. 2011, 184, 2478–2484. [Google Scholar] [CrossRef]
- Sharma, B.B.; Parashar, A. A review on thermo-mechanical properties of bi-crystalline and polycrystalline 2D nanomaterials. Crit. Rev. Solid State Mater. Sci. 2019, 45, 134–170. [Google Scholar] [CrossRef]
- Yu, B.-S.; Ha, T.-J. High-performance Solution-Processed IGZO Thin-Film Transistors with Al2O3/BN Composite dielectrics Fabricated at Low Temperature. Phys. Status Solidi A 2018, 215, 1700802. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Chong, J.Y.; Wang, B.; Mattevi, C.; Li, K. Dynamic microstructure of graphene oxide membranes and permeation flux. J. Membr. Sci. 2018, 549, 385–392. [Google Scholar] [CrossRef]
- Fortunato, E.; Barquinha, P.; Martins, R. Oxide Semiconductor Thin-Film Transistors: A Review of Recent Advances. Adv. Mater. 2012, 24, 2945–2986. [Google Scholar] [CrossRef]
- Atkinson, J.D.; Fortunato, M.A.; Dastgheib, S.A.; Rostam-Abadia, M.; Rooda, M.J.; Suslick, K.S. Synthesis and characterization of iron-impregnated porous carbon spheres by ultrasonic spray pyrolysis. Carbon 2011, 49, 587–598. [Google Scholar] [CrossRef]
- Kim, H.; Fortunato, M.E.; Xu, H.; Bang, J.H.; Suslick, K.S. Carbon microspheres as Supercapacitors. J. Phys. Chem. C 2011, 115, 20481–20486. [Google Scholar] [CrossRef]
- Motuma, B.K.; Matoso, B.J.; Momodu, D.; Oyedotun, K.O.; Coville, N.J.; Manyala, N. Deciphering the Structural, Textural, and Electrochemical Properties of Activated BN-Doped Spherical Carbons. Nanomaterials 2019, 9, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, H.; Yang, G.; Zhao, B.; Yang, J. Catalyst-free synthesis of multi-walled carbon nanotubes from carbon spheres and its implications for the formation mechanism. Carbon 2013, 53, 137–144. [Google Scholar] [CrossRef]
- Lobo, L.S. Mechanism of catalytic CNTs growth in 400–650 °C range: Explaining volcano shape Arrhenius plot and catalytic synergism using both Pt (or Pd) and Ni, Co or Fe. C-J. Carb. Res. 2019, 5, 42. [Google Scholar] [CrossRef] [Green Version]








| Kinetic Routes | Temperature Range (°C) | C Growth Type | Active Catalysts |
|---|---|---|---|
| I Catalytic | 300–550 | Surface catalysis | Fe, Co, Ni |
| II Hybrid | 550–(700) | C black atoms dissolve/grow | Pt, Ru, Mo, Ni, Cu |
| III Pyrolytic | 600–(1200) | C black (C2/C3) forms layers | No catalysis, shape adjusts |
| Metal Solvent | Melting (°C) | TTa (°C) | ARsolv (pm) | ARsoluC/ARsolv | ARsoluN/ARsolv | ARsoluB/ARsolv |
|---|---|---|---|---|---|---|
| Fe | 1538 | 632 | 140 | 0.50 | 0.36 | 0.56 |
| Co | 1495 | 611 | 135 | 0.52 | 0.37 | 0.57 |
| Ni | 1455 | 590 | 135 | 0.52 | 0.38 | 0.58 |
| Cu | 1083 | 405 | 135 | 0.52 | 0.39 | 0.60 |
| Ru | 2334 | 1030 | 130 | 0.54 | 0.31 | 0.49 |
| Rh | 1964 | 845 | 135 | 0.52 | 0.32 | 0.50 |
| Pd | 1555 | 641 | 140 | 0.50 | 0.33 | 0.51 |
| Catalyst | Gas | Temperature (°C) | First Author | Year | Reference |
|---|---|---|---|---|---|
| Ni-Cu/Al | CH4/N2 | 500–730 | Li YD | 1999 | [18] |
| Fe/SiO2 | C2H2 | 750–950 | Lee CJ | 2000 | [7] |
| Fe/SiO2 | CH4/NH3 | 650–950 | Cui H | 2000 | [19] |
| Ni/Al | CH4/N2 | 500–600 | He C | 2007 | [20] |
| Ni-Cu/Al2O3 | CH4/H2 | 720–830 | Chen J | 2001 | [21] |
| Ni | C2H2/N2/H2 | 750–950 | Jung M | 2001 | [22] |
| Ni | Phthalocyanine | 600–850 | Katayama T | 2002 | [23] |
| Fe | Phthalocyanine | 1000 | Chadderton LT | 2002 | [24] |
| Fe,Co,Ni | CH4/H2 | 850–1100 | Bartsch K | 2005 | [25] |
| Co/Al2O3-Ti | C2H2/NH3 | 750–950 | Jang JY | 2006 | [26] |
| Fe | C2H2/NH3/H2 | 700 | Ting JM | 2007 | [27] |
| Ni | C2H2 | 650 | Lin MT | 2007 | [28] |
| Cu | CH4/H2/H2S | 500–900 | Katar SL | 2008 | [29] |
| Cu/Al2O3 | C2H5OH | 700–850 | Xue B | 2009 | [30] |
| Ni (AC)2 | C4H4S/H2-S | Detonation | Wang C | 2010 | [31] |
| Ni, Ni-Cu | CH4/N2 | 550–830 | Gonzalez I | 2011 | [9] |
| Pt/SiO2 | CH4/NH3 | 1000/Plasma | Brown B | 2011 | [8] |
| Cu/Al2O3 | C2H4/He | 700–900 | Lin JH | 2012 | [32] |
| Cu | Ethanol | 700–1000 | Zhu J | 2012 | [33] |
| Fe,Co,Ni,Al2O3 | C2H2 | 720 | Keczenovity E | 2013 | [34] |
| Cu/SiO2 | C2H4/He | 500–900 | Lin YC | 2013 | [35] |
| Cu/Al2O3 | C2H2/N2 | 550–800 | Krishna VM | 2014 | [36] |
| La/NiO3 | Glicerol/Ethanol | 700–900 | Velasquez M | 2014 | [37] |
| Ferrocene/SiO2 | Dichlorobenzene | 800–900 | Boi FS | 2016 | [38] |
| Ni,Cu,Zn | CH4 | 600–800 | Saraswat SK | 2016 | [39] |
| Fe-Mo/Al2O3 | C3H4N2 | 800–900 | Wang Q | 2017 | [40] |
| Fe/Al2O3 | Polyamide | 750 | Arnaiz N | 2018 | [41] |
| Cobaltocene | Ethanol | 500 | Tang Y | 2018 | [42] |
| Co-Fe/Ru | CO/H2 | 750 | Kumi DO | 2018 | [43] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobo, L.S.; Carabineiro, S.A.C. Explaining Bamboo-Like Carbon Fiber Growth Mechanism: Catalyst Shape Adjustments above Tammann Temperature. C 2020, 6, 18. https://doi.org/10.3390/c6020018
Lobo LS, Carabineiro SAC. Explaining Bamboo-Like Carbon Fiber Growth Mechanism: Catalyst Shape Adjustments above Tammann Temperature. C. 2020; 6(2):18. https://doi.org/10.3390/c6020018
Chicago/Turabian StyleLobo, Luís Sousa, and Sónia A.C. Carabineiro. 2020. "Explaining Bamboo-Like Carbon Fiber Growth Mechanism: Catalyst Shape Adjustments above Tammann Temperature" C 6, no. 2: 18. https://doi.org/10.3390/c6020018
APA StyleLobo, L. S., & Carabineiro, S. A. C. (2020). Explaining Bamboo-Like Carbon Fiber Growth Mechanism: Catalyst Shape Adjustments above Tammann Temperature. C, 6(2), 18. https://doi.org/10.3390/c6020018