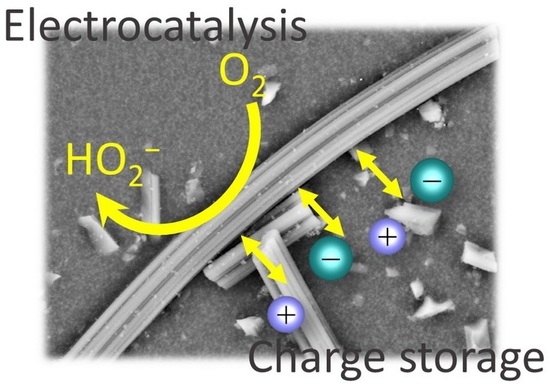
Exploring the Impact of DAHP Impregnation on Activated Carbon Fibers for Efficient Charge Storage and Selective O2 Reduction to Peroxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis and Characterization
2.2. Capacitance Measurements
2.3. ORR Measurements
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Li, C.; Cao, F.; Noori, A.; Mousavi, M.F.; Xia, X. Carbon in Electrochemical Energy. Mater. Res. Bull. 2022, 152, 111852. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, K.; Wang, Z. A Review of the Application of Carbon Materials for Lithium Metal Batteries. Batteries 2022, 8, 246. [Google Scholar] [CrossRef]
- Yang, S.; Cheng, Y.; Xiao, X.; Pang, H. Development and Application of Carbon Fiber in Batteries. Chem. Eng. J. 2020, 384, 123294. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent Progress in Carbon-Based Materials for Supercapacitor Electrodes: A Review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A Review of Carbon Materials for Supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Jaleh, B.; Nasrollahzadeh, M.; Eslamipanah, M.; Nasri, A.; Shabanlou, E.; Manwar, N.R.; Zboril, R.; Fornasiero, P.; Gawande, M.B. The Role of Carbon-Based Materials for Fuel Cells Performance. Carbon 2022, 198, 301–352. [Google Scholar] [CrossRef]
- Hadadian, M.; Smått, J.-H.; Correa-Baena, J.-P. The Role of Carbon-Based Materials in Enhancing the Stability of Perovskite Solar Cells. Energy Environ. Sci. 2020, 13, 1377–1407. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Kim, J. A Review on Application of Activated Carbons for Carbon Dioxide Capture: Present Performance, Preparation, and Surface Modification for Further Improvement. Environ. Sci. Pollut. Res. 2021, 28, 43329–43364. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Lu, X.; Wang, D. A Systematic Review of Carbon Capture, Utilization and Storage: Status, Progress and Challenges. Energies 2023, 16, 2865. [Google Scholar] [CrossRef]
- Bader, N.; Ouederni, A. Optimization of Biomass-Based Carbon Materials for Hydrogen Storage. J. Energy Storage 2016, 5, 77–84. [Google Scholar] [CrossRef]
- Mohan, M.; Sharma, V.K.; Kumar, E.A.; Gayathri, V. Hydrogen Storage in Carbon Materials—A Review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Pang, T.; Marken, F.; Zhang, D.; Mattia, D.; Shen, J. Linking Macroscopic Surface Morphology of Activated Carbon Fibres and Electrosorption Performance: An Electrochemical Impedance Spectroscopy and Capacitive Deionization Study. Appl. Surf. Sci. 2023, 609, 155397. [Google Scholar] [CrossRef]
- Pašti, I.A.; Janošević Ležaić, A.; Gavrilov, N.M.; Ćirić-Marjanović, G.; Mentus, S.V. Nanocarbons Derived from Polymers for Electrochemical Energy Conversion and Storage—A Review. Synth. Met. 2018, 246, 267–281. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed]
- Rani, J.; Thangavel, R.; Oh, S.-I.; Lee, Y.; Jang, J.-H. An Ultra-High-Energy Density Supercapacitor; Fabrication Based on Thiol-Functionalized Graphene Oxide Scrolls. Nanomaterials 2019, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Felhősi, I.; Keresztes, Z.; Marek, T.; Pajkossy, T. Properties of Electrochemical Double-Layer Capacitors with Carbon-Nanotubes-on-Carbon-Fiber-Felt Electrodes. Electrochim. Acta 2020, 334, 135548. [Google Scholar] [CrossRef]
- Frackowiak, E.; Jurewicz, K.; Delpeux, S.; Beguin, F. Electrochemical Application of Carbon Nanotubes. In Low-Dimensional Systems: Theory, Preparation, and Some Applications; Springer: Dordrecht, The Netherlands, 2003; pp. 305–318. [Google Scholar]
- Chen, J.; Li, C.; Shi, G. Graphene Materials for Electrochemical Capacitors. J. Phys. Chem. Lett. 2013, 4, 1244–1253. [Google Scholar] [CrossRef]
- Udayakumar, M.; Tóth, P.; Wiinikka, H.; Malhotra, J.S.; Likozar, B.; Gyergyek, S.; Leskó, A.K.; Thangaraj, R.; Németh, Z. Hierarchical Porous Carbon Foam Electrodes Fabricated from Waste Polyurethane Elastomer Template for Electric Double-Layer Capacitors. Sci. Rep. 2022, 12, 11786. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Feng, Y.; Tian, R.; Chen, Q.; Chen, J.; Jia, M.; Yao, J. Free-Standing Porous Carbon Foam as the Ultralight and Flexible Supercapacitor Electrode. Carbon 2020, 161, 224–230. [Google Scholar] [CrossRef]
- Park, H.; Seo, J.; Kim, M.; Baeck, S.-H.; Shim, S.E. Development of a Carbon Foam Supercapacitor Electrode from Resorcinol–Formaldehyde Using a Double Templating Method. Synth. Met. 2015, 199, 121–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, H.; Zhou, Q.; Liu, K.; Ma, W.; Fan, S. Biomass-Derived Carbon for Supercapacitors Electrodes—A Review of Recent Advances. Inorg. Chem. Commun. 2023, 153, 110768. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon Materials as Catalyst Supports and Catalysts in the Transformation of Biomass to Fuels and Chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Rodŕíguez-Reinoso, F.; Seṕulveda-Escribano, A. Carbon as Catalyst Support. In Carbon Materials for Catalysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 131–155. [Google Scholar]
- Trogadas, P.; Fuller, T.F.; Strasser, P. Carbon as Catalyst and Support for Electrochemical Energy Conversion. Carbon 2014, 75, 5–42. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A Review of Oxygen Reduction Mechanisms for Metal-Free Carbon-Based Electrocatalysts. NPJ Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Taguchi, T.; Gohda, S.; Gotoh, K.; Sato, S.; Yamada, Y. Synthesis of Carbon Materials with Extremely High Pyridinic-Nitrogen Content and Controlled Edges from Aromatic Compounds with Highly Symmetric Skeletons. Carbon Lett. 2023, 33, 1279–1301. [Google Scholar] [CrossRef]
- Pašti, I.A.; Gavrilov, N.M.; Dobrota, A.S.; Momčilović, M.; Stojmenović, M.; Topalov, A.; Stanković, D.M.; Babić, B.; Ćirić-Marjanović, G.; Mentus, S.V. The Effects of a Low-Level Boron, Phosphorus, and Nitrogen Doping on the Oxygen Reduction Activity of Ordered Mesoporous Carbons. Electrocatalysis 2015, 6, 498–511. [Google Scholar] [CrossRef]
- Tuci, G.; Rossin, A.; Zhang, X.; Truong-Phuoc, L.; Berretti, E.; Liu, Y.; Pham-Huu, C.; Ali, S.; Jan, F.; Poggini, L.; et al. Metal-Free Electrocatalysts for the Selective 2 e− Oxygen Reduction Reaction: A Never-Ending Story? Chem.—Eur. J. 2023, 29, e202301036. [Google Scholar] [CrossRef] [PubMed]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process. Angew. Chemie Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Foller, P.C.; Bombard, R.T. Processes for the Production of Mixtures of Caustic Soda and Hydrogen Peroxide via the Reduction of Oxygen. J. Appl. Electrochem. 1995, 25, 613–627. [Google Scholar] [CrossRef]
- Gopal, R. Electrochemical Synthesis of Hydrogen Peroxide. U.S. Patent 20030019758A1, 30 March 2004. [Google Scholar]
- Perry, S.C.; Pangotra, D.; Vieira, L.; Csepei, L.-I.; Sieber, V.; Wang, L.; Ponce de León, C.; Walsh, F.C. Electrochemical Synthesis of Hydrogen Peroxide from Water and Oxygen. Nat. Rev. Chem. 2019, 3, 442–458. [Google Scholar] [CrossRef]
- Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Čolić, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I.E.L. Toward the Decentralized Electrochemical Production of H2O2: A Focus on the Catalysis. ACS Catal. 2018, 8, 4064–4081. [Google Scholar] [CrossRef]
- Pizzutilo, E.; Kasian, O.; Choi, C.H.; Cherevko, S.; Hutchings, G.J.; Mayrhofer, K.J.J.; Freakley, S.J. Electrocatalytic Synthesis of Hydrogen Peroxide on Au-Pd Nanoparticles: From Fundamentals to Continuous Production. Chem. Phys. Lett. 2017, 683, 436–442. [Google Scholar] [CrossRef]
- Huang, X.; Song, M.; Zhang, J.; Shen, T.; Luo, G.; Wang, D. Recent Advances of Electrocatalyst and Cell Design for Hydrogen Peroxide Production. Nano-Micro Lett. 2023, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, J.; Chen, Z.; Yang, H.; Zou, K.; Zhao, X.; Liang, R.; Dong, X.; Menezes, P.W.; Kang, Z. Entropy Enhanced Perovskite Oxide Ceramic for Efficient Electrochemical Reduction of Oxygen to Hydrogen Peroxide. Angew. Chem. 2022, 134, e202200086. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, X.; Xia, C.; Wu, Z.-Y.; Zhu, P.; Kim, J.Y.; Bai, X.; Gao, G.; Hu, Y.; Zhong, J.; et al. Highly Active and Selective Oxygen Reduction to H2O2 on Boron-Doped Carbon for High Production Rates. Nat. Commun. 2021, 12, 4225. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Xia, Y.; Zhu, P.; Fan, L.; Wang, H. Direct Electrosynthesis of Pure Aqueous H2O2 Solutions up to 20% by Weight Using a Solid Electrolyte. Science 2019, 366, 226–231. [Google Scholar] [CrossRef]
- Kim, H.W.; Ross, M.B.; Kornienko, N.; Zhang, L.; Guo, J.; Yang, P.; McCloskey, B.D. Efficient Hydrogen Peroxide Generation Using Reduced Graphene Oxide-Based Oxygen Reduction Electrocatalysts. Nat. Catal. 2018, 1, 282–290. [Google Scholar] [CrossRef]
- An, J.; Feng, Y.; Zhao, Q.; Wang, X.; Liu, J.; Li, N. Electrosynthesis of H2O2 through a Two-Electron Oxygen Reduction Reaction by Carbon Based Catalysts: From Mechanism, Catalyst Design to Electrode Fabrication. Environ. Sci. Ecotechnol. 2022, 11, 100170. [Google Scholar] [CrossRef]
- Jocić, A.; Breitenbach, S.; Bajuk-Bogdanović, D.; Pašti, I.A.; Unterweger, C.; Fürst, C.; Lazarević-Pašti, T. Viscose-Derived Activated Carbons Fibers as Highly Efficient Adsorbents for Dimethoate Removal from Water. Molecules 2022, 27, 1477. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental Methods in Chemical Engineering: Specific Surface Area and Pore Size Distribution Measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Kupgan, G.; Liyana-Arachchi, T.P.; Colina, C.M. NLDFT Pore Size Distribution in Amorphous Microporous Materials. Langmuir 2017, 33, 11138–11145. [Google Scholar] [CrossRef] [PubMed]
- Jocić, A.; Breitenbach, S.; Pašti, I.A.; Unterweger, C.; Fürst, C.; Lazarević-Pašti, T. Viscose-Derived Activated Carbons as Adsorbents for Malathion, Dimethoate, and Chlorpyrifos—Screening, Trends, and Analysis. Environ. Sci. Pollut. Res. 2022, 29, 35138–35149. [Google Scholar] [CrossRef]
- Breitenbach, S.; Gavrilov, N.; Pašti, I.; Unterweger, C.; Duchoslav, J.; Stifter, D.; Hassel, A.W.; Fürst, C. Biomass-Derived Carbons as Versatile Materials for Energy-Related Applications: Capacitive Properties vs. Oxygen Reduction Reaction Catalysis. C 2021, 7, 55. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Milakin, K.A.; Gavrilov, N.; Pašti, I.A.; Morávková, Z.; Acharya, U.; Unterweger, C.; Breitenbach, S.; Zhigunov, A.; Bober, P. Polyaniline-Metal Organic Framework (Fe-BTC) Composite for Electrochemical Applications. Polymer 2020, 208, 122945. [Google Scholar] [CrossRef]
- Vujković, M.; Gavrilov, N.; Pašti, I.; Krstić, J.; Travas-Sejdic, J.; Ćirić-Marjanović, G.; Mentus, S. Superior Capacitive and Electrocatalytic Properties of Carbonized Nanostructured Polyaniline upon a Low-Temperature Hydrothermal Treatment. Carbon 2013, 64, 472–486. [Google Scholar] [CrossRef]
- Gavrilov, N.; Pašti, I.A.; Vujković, M.; Travas-Sejdic, J.; Ćirić-Marjanović, G.; Mentus, S.V. High-Performance Charge Storage by N-Containing Nanostructured Carbon Derived from Polyaniline. Carbon 2012, 50, 3915–3927. [Google Scholar] [CrossRef]
- Gavrilov, N.; Vujković, M.; Pašti, I.A.; Ćirić-Marjanović, G.; Mentus, S.V. Enhancement of Electrocatalytic Properties of Carbonized Polyaniline Nanoparticles upon a Hydrothermal Treatment in Alkaline Medium. Electrochim. Acta 2011, 56, 9197–9202. [Google Scholar] [CrossRef]
- Breitenbach, S.; Unterweger, C.; Lumetzberger, A.; Duchoslav, J.; Stifter, D.; Hassel, A.W.; Fürst, C. Viscose-based Porous Carbon Fibers: Improving Yield and Porosity through Optimization of the Carbonization Process by Design of Experiment. J. Porous Mater. 2021, 28, 727–739. [Google Scholar] [CrossRef]
- Breitenbach, S.; Lumetzberger, A.; Hobisch, M.A.; Unterweger, C.; Spirk, S.; Stifter, D.; Fürst, C.; Hassel, A.W. Supercapacitor Electrodes from Viscose-Based Activated Carbon Fibers: Significant Yield and Performance Improvement Using Diammonium Hydrogen Phosphate as Impregnating Agent. C 2020, 6, 17. [Google Scholar] [CrossRef]





| X= | 0.00% | 0.25% | 0.50% | 1.00% | 1.50% | 2.00% | 2.50% | 5.00% | 7.50% | 10% | |
| V1nm/cm3 g−1 | 0.348 | 0.335 | 0.339 | 0.343 | 0.297 | 0.271 | 0.274 | 0.198 | 0.137 | 0.160 | |
| Vtot cm3 g−1 | 0.757 | 0.383 | 0.472 | 0.774 | 0.770 | 1.094 | 0.833 | 1.291 | 1.681 | 1.322 | |
| Stot/m2 g−1 | 1932 | 1016 | 1250 | 2037 | 2002 | 2556 | 2018 | 2718 | 2763 | 2718 | |
| Elemental Content | C/at.% | 92.4 ± 2.1 | 91.6 ± 3.5 | 93.6 ± 2.3 | 87.9 ± 1.3 | 93.9 ± 1.9 | 91.3 ± 2.0 | 87.9 ± 2.0 | 85.6 ± 2.5 | 82.2 ± 2.2 | 77.8 ± 5.0 |
| O/at.% | 7.6 ± 2.0 | 8.4 ± 3.5 | 6.2 ± 2.2 | 12 ± 1.3 | 5.9 ± 1.9 | 7.7 ± 2.3 | 11.4 ± 2.2 | 13.1 ± 2.7 | 16.1 ± 2.3 | 19.7 ± 5.3 | |
| P/at.% | 0 | 0.02 ± 0.02 | 0.18 ± 0.10 | 0.11 ± 0.04 | 0.28 ± 0.07 | 0.91 ± 0.29 | 0.65 ± 0.30 | 1.32 ± 0.34 | 1.78 ± 0.13 | 1.9 ± 0.64 | |
| Material | 5 mV s−1 | 10 mV s−1 | 20 mV s−1 | 50 mV s−1 | 100 mV s−1 | 200 mV s−1 | 400 mV s−1 |
|---|---|---|---|---|---|---|---|
| 0.0% DAHP | 84.5 ± 6.8 | 87.6 ± 6.1 | 89.4 ± 6.3 | 87.2 ± 5.2 | 75.5 ± 4.5 | 69.1 ± 4.1 | 59.5 ± 4.2 |
| 0.25% DAHP | 94.6 ± 7.6 | 88.4 ± 4.4 | 82.2 ± 4.1 | 70.9 ± 4.3 | 56.6 ± 3.4 | 46.0 ± 2.8 | 36.6 ± 2.9 |
| 0.5% DAHP | 87.8 ± 7.9 | 78.1 ± 6.2 | 75.8 ± 4.5 | 74.9 ± 3.0 | 62.6 ± 2.5 | 57.7 ± 2.3 | 51.9 ± 2.6 |
| 1.0% DAHP | 133 ± 7 | 149 ± 10 | 168.2 ± 6.7 | 174 ± 11 | 159 ± 8 | 151 ± 11 | 138 ± 11 |
| 1.5% DAHP | 192 ± 8 | 137.3 ± 6.9 | 144 ± 7 | 148.0 ± 5.9 | 135.0 ± 8.1 | 128.2 ± 6.4 | 118.1 ± 9.4 |
| 2.0% DAHP | 125 ± 11 | 146 ± 6 | 168 ± 7 | 173 ± 7 | 156.2 ± 7.8 | 150 ± 14 | 138 ± 11 |
| 2.5% DAHP | 141.9 ± 8.5 | 152.2 ± 6.1 | 166 ± 10 | 167 ± 10 | 153.0 ± 6.1 | 142.8 ± 5.7 | 128.9 ± 6.4 |
| 5.0% DAHP | 92.5 ± 7.4 | 93.0 ± 7.4 | 113.0 ± 4.5 | 126.0 ± 6.3 | 112.7 ± 7.9 | 112.4 ± 7.9 | 107.4 ± 7.5 |
| 7.5% DAHP | 91.4 ± 3.7 | 77.8 ± 6.2 | 84.2 ± 5.9 | 88.9 ± 5.3 | 78.3 ± 4.7 | 75.2 ± 6.0 | 69.7 ± 4.9 |
| 10.0% DAHP | 124.7 ± 7.5 | 107.3 ± 6.4 | 110.7 ± 6.6 | 115.4 ± 8.1 | 99.8 ± 4.0 | 96.9 ± 8.7 | 91.5 ± 6.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrilov, N.; Breitenbach, S.; Unterweger, C.; Fürst, C.; Pašti, I.A. Exploring the Impact of DAHP Impregnation on Activated Carbon Fibers for Efficient Charge Storage and Selective O2 Reduction to Peroxide. C 2023, 9, 105. https://doi.org/10.3390/c9040105
Gavrilov N, Breitenbach S, Unterweger C, Fürst C, Pašti IA. Exploring the Impact of DAHP Impregnation on Activated Carbon Fibers for Efficient Charge Storage and Selective O2 Reduction to Peroxide. C. 2023; 9(4):105. https://doi.org/10.3390/c9040105
Chicago/Turabian StyleGavrilov, Nemanja, Stefan Breitenbach, Christoph Unterweger, Christian Fürst, and Igor A. Pašti. 2023. "Exploring the Impact of DAHP Impregnation on Activated Carbon Fibers for Efficient Charge Storage and Selective O2 Reduction to Peroxide" C 9, no. 4: 105. https://doi.org/10.3390/c9040105
APA StyleGavrilov, N., Breitenbach, S., Unterweger, C., Fürst, C., & Pašti, I. A. (2023). Exploring the Impact of DAHP Impregnation on Activated Carbon Fibers for Efficient Charge Storage and Selective O2 Reduction to Peroxide. C, 9(4), 105. https://doi.org/10.3390/c9040105