Probiotic Functional Yogurt: Challenges and Opportunities
Abstract
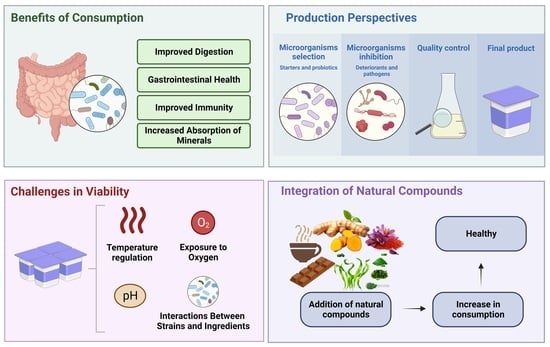
:1. Introduction
2. Yogurts and Functional Foods: A Contemporary Perspective
Probiotic Microorganisms
3. Probiotic Functional Yogurts Enriched with Natural Compounds
4. Aspects Related to the Fermentation of Probiotic Functional Yogurt
4.1. Technical Challenges Associated with Probiotic Functional Yogurt Fermentation
4.2. Challenges in Maintaining Viability and Microbiological Quality of Yogurt
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelhamid, S.M.; Edris, A.E.; Sadek, Z. Novel approach for the inhibition of Helicobacter pylori contamination in yogurt using selected probiotics combined with eugenol and cinnamaldehyde nanoemulsions. Food Chem. 2023, 417, 135877. [Google Scholar] [CrossRef] [PubMed]
- Demirkol, M.; Tarakci, Z. Effect of grape (Vitis labrusca L.) pomace dried by different methods on physicochemical, microbiological and bioactive properties of yoghurt. LWT 2018, 97, 770–777. [Google Scholar] [CrossRef]
- Maharani, M.B.S.; Soviana, S.; Pisestyani, H. Examination of milk quality from milk shops in the residential areas of IPB dramaga and Cilibende. J. Kaj. Veter. 2020, 8, 24–33. [Google Scholar]
- Ribeiro, B.D.; Do Nascimento, R.P.; Pereira, K.S.; Coelho, M.A.Z. Microbiologia Industrial: Alimentos; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 329–370. [Google Scholar]
- Deshwal, G.K.; Tiwari, S.; Kumar, A.; Raman, R.K.; Kadyan, S. Review on factors affecting and control of post-acidification in yoghurt and related products. Trends Food Sci. Technol. 2021, 109, 499–512. [Google Scholar] [CrossRef]
- Cooper, G. Food Microbiology; Larsen and Keller Education: New York, NY, USA, 2019. [Google Scholar]
- Tewari, S.; David, J.P.; Gautam, A. A review on probiotic dairy products and digestive health. J. Pharmacogn. Phytochem. 2019, 8, 368–372. [Google Scholar]
- Arab, M.; Sohrabvandi, S.; Khorshidian, N.; Mortazavian, A.M. Combined effects of salt-related variables on qualitative characteristics of probiotic fermented milk. Curr. Nutr. Food Sci. 2019, 15, 234–242. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kanellaki, M.; Kopsahelis, N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT 2019, 105, 242–249. [Google Scholar] [CrossRef]
- Fazilah, N.F.; Arif, A.B.; Khayat, M.E.; Rios-Solis, L.; Halim, M. Influence of probiotics, prebiotics, synbiotics and bioactive phytochemicals on the formulation of functional yogurt. J. Funct. Foods 2018, 48, 387–399. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Balthazar, C.F.; Silva, R.; Rocha, R.S.; Graça, J.S.; Esmerino, E.A.; Silva, M.C.; Sant’Ana, A.S.; Duarte, M.C.K.H.; Freitas, M.Q.; et al. Impact of probiotics and prebiotics on food texture. Curr. Opin. Food Sci. 2020, 33, 38–44. [Google Scholar] [CrossRef]
- Luo, H.; Bao, Y.; Zhu, P. Development of a novel functional yogurt rich in lycopene by Bacillus subtilis. Food Chem. 2023, 407, 135142. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Z.; Zhang, Q.; Yu, L.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Effects of Bacillus coagulans GBI-30, 6086 as an adjunct starter culture on the production of yogurt. Food Res. Int. 2022, 160, 111398. [Google Scholar] [CrossRef] [PubMed]
- Niamah, A.K. Physicochemical and Microbial Characteristics of Yogurt with Added Saccharomyces Boulardii. Curr. Res. Nutr. Food. Sci. 2017, 5, 300–307. [Google Scholar] [CrossRef]
- Sarwar, A.; Aziz, T.; Al-Dalali, S.; Zhao, X.; Zhang, J.; Ud Din, J.; Chen, C.; Cao, Y.; Yang, Z. Physicochemical and Microbiological Properties of Synbiotic Yogurt Made with Probiotic Yeast Saccharomyces boulardii in Combination with Inulin. Foods 2019, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Barengolts, E.; Smith, E.D.; Reutrakul, S.; Tonucci, L.; Anothaisintawee, T. The effect of probiotic yogurt on glycemic control in type 2 diabetes or obesity: A meta-analysis of nine randomized controlled trials. Nutrients 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh, H.; Yavarmanesh, M.; Mortazavi, S.A.; Adibi, P.; Moazzami, A.A. Metabolomics analysis revealed metabolic changes in patients with diarrhea-predominant irritable bowel syndrome and metabolic responses to a synbiotic yogurt intervention. Eur. J. Nutr. 2019, 58, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.Y.; How, H.; Pui, L.P. Microencapsulation of Bifidobacterium lactis Bi-07 with galaactooligosaccharides using co-extrusion technique. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e2416. [Google Scholar]
- Ballini, A.; Charitos, I.A.; Cantore, S.; Topi, S.; Bottalico, L.; Santacroce, L. About Functional Foods: The Probiotics and Prebiotics State of Art. Antibiotics 2023, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Investigating the Effect of Consumers Knowledge on Their Acceptance of Functional Foods: A Systematic Review and Meta-Analysis. Foods 2022, 11, 1135. [Google Scholar] [CrossRef]
- Meybodi, N.M.; Mortazavian, A.M.; Arab, M.; Nematollahi, A. Probiotic viability in yoghurt: A review of influential factors. Int. Dairy J. 2020, 109, 104793. [Google Scholar] [CrossRef]
- Fuller, R. Probiotics: The Scientific Basis; Chapman and Hall: London, UK, 1992. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merestein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Champagne, C.P.; Da Cruz, A.G.; Daga, M. Strategies to improve the functionality of probiotics in supplements and foods. Curr. Opin. Food Sci. 2018, 22, 160–166. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Laursen, R.R.; Ouwehand, A.C. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sekhon, A.S.; Unger, P.; Lampien, A.; Galland, A.T.; Bhavnani, K.; Michael, M. Impact of ultrafine bubbles on the survivability of probiotics in fermented milks. Int. Dairy J. 2023, 140, 122–131. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Da Costa, W.K.A.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2021, 140, 110–133. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Bhunia, A. Fundamental Food Microbiology, 5th ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Schillinger, U. Isolation and identification of lactobacilli from novel-type probiotic and mild yoghurts and their stability during refrigerated storage. Int. J. Food Microbiol. 1999, 47, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Espinoza, Y.; Gallardo-Navarro, Y. Non-dairy probiotic products. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Forssten, S.D.; Sindelar, C.W.; Ouwehand, A.C. Probiotics from an industrial perspective. Anaerobe 2011, 17, 410–413. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Kariyawasam, M.G.M.; Kariyawasam, M.; Lee, N.; Paik, H. Synbiotic yoghurt supplemented with novel probiotic Lactobacillus brevis KU200019 and fructooligosaccharides. Food Biosci. 2021, 39, 100835. [Google Scholar] [CrossRef]
- Soccol, C.R.; Vandenberghe, L.P.S.; Spier, M.R.; Medeiros, A.B.P.; Yamaguishi, C.T.; Lindner, J.D.; Pandey, A.; Thomaz-Soccol, V. The potential of probiotics: A review. Food Technol. Biotechnol. 2010, 48, 413–434. [Google Scholar]
- Pei, Z.; Sadiq, F.A.; Han, X.; Zhao, J.; Zhang, H.; Ross, R.P.; Lu, W.; Chen, W. Identification, characterization, and phylogenetic analysis of eight new inducible prophages in Lactobacillus. Virus Res. 2020, 286, 198003. [Google Scholar] [CrossRef] [PubMed]
- Westerik, N.; Kort, R.; Sybesma, W.; Reid, G. Lactobacillus rhamnosus probiotic foods as a training tool across the value chain in Africa. Front. Microbiol. 2018, 9, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kemgang, T.S.; Kapila, S.; Shanmugam, P.V.; Reddi, S.; Kapila, R. Leite fermentado com probiótico Lactobacillus rhamnosus S1K3 (MTCC5957) protege camundongos da salmonela aumentando os mecanismos de proteção imune e não imune no nível da mucosa intestinal. J. Nutr. Biochem. 2016, 30, 62–73. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Li, J.; Wang, H.; Zhang, Y.; Suo, H. Lactobacillus rhamnosus 2016SWU.05.0601 regulates immune balance in ovalbumin-sensitized mice by modulating expression of the immune-related transcription factors and gut microbiota. J. Sci. Food Agric. 2020, 100, 4930–4939. [Google Scholar] [CrossRef]
- Forsythe, S.J. Microbiologia da Segurança dos Alimentos; Artmed: Porto Alegre, Brazil, 2013. [Google Scholar]
- Shah, N.P.; Lankaputhra, W.E.V. Bifidobacterium spp.: Morphology and physiology. Encycl. Dairy Sci. 2002, 141–146. [Google Scholar] [CrossRef]
- Trabulsi, L.R.; Alterthum, F. Microbiologia, 6th ed.; Editora Atheneu: São Paulo, Brazil, 2015. [Google Scholar]
- Lee, N.K.; Kim, W.S.; Paik, H.D. Bacillus strains as human probiotics: Characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef]
- Todorov, S.D.; Ivanova, I.V.; Popov, I.; Weeks, R.; Chikindas, M.L. Bacillus spore-forming probiotics: Benefits with concerns? Crit. Rev. Microbiol. 2022, 48, 513–530. [Google Scholar] [CrossRef]
- Ansari, F.; Samakkhah, S.A.; Bahadori, A.; Jafari, S.M.; Ziaee, M.; Khodayari, M.T.; Pourjafar, H. Health-promoting properties of Saccharomyces cerevisiae var. boulardii as a probiotic; characteristics, isolation, and applications in dairy products. Crit. Rev. Food Sci. Nutr. 2021, 63, 457–485. [Google Scholar] [CrossRef]
- Zúñiga, M.; Monedero, V.; Yebra, M.J. Utilization of Host-Derived Glycans by Intestinal Lactobacillus and Bifidobacterium Species. Front. Microbiol. 2018, 9, 1917. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Duan, Y.; Xu, Z.; Lyu, N.; Liu, F.; Liang, S.; Zhu, B. An examination of data from the American Gut Project reveals that the dominance of the genus Bifidobacterium is associated with the diversity and robustness of the gut microbiota. MicrobiologyOpen 2019, 8, e939. [Google Scholar] [CrossRef]
- James, A.; Wang, Y. Characterization, health benefits and applications of fruits and vegetable probiotics. CYTA J. Food 2019, 17, 770–780. [Google Scholar] [CrossRef]
- Chugh, B.; Kamal-Eldin, A. Bioactive Compounds Produced by Probiotics in Food Products. Curr. Opin. Food Sci. 2020, 32, 76–82. [Google Scholar] [CrossRef]
- Delgado, S.; Sánchez, B.; Margolles, A.; Ruas-Madiedo, P.; Ruiz, L. Molecules Produced by Probiotics and Intestinal Microorganisms with Immunomodulatory Activity. Nutrients 2020, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- Parvarei, M.M.; Khorshidian, N.; Fazeli, M.R.; Mortazavian, A.M.; Nezhad, S.S.; Mortaza, S.A. Comparative effect of probiotic and paraprobiotic addition on physicochemical, chemometric and microstructural properties of yogurt. LWT 2021, 144, 111177. [Google Scholar] [CrossRef]
- Widyastuti, Y.; Febrisiantosa, A.; Tidona, F. Health-Promoting Properties of Lactobacilli in Fermented Dairy Products. Front Microbiol. 2021, 12, 673890. [Google Scholar] [CrossRef]
- Khalifa, A.; Sheikh, A.; Ibrahim, H.I.M. Bacillus amyloliquefaciens Enriched Camel Milk Attenuated Colitis Symptoms in Mice Model. Nutrients 2022, 14, 1967. [Google Scholar] [CrossRef]
- Almada-Érix, C.N.; Almada, C.N.; Cabral, L.; Barros de Medeiros, V.P.; Roquetto, A.R.; Santos-Junior, V.A.; Fontes, M.; Gonçalves, A.E.S.S.; dos Santos, A.; Lollo, P.C.; et al. Orange Juice and Yogurt Carrying Probiotic Bacillus coagulans GBI-30 6086: Impact of Intake on Wistar Male Rats Health Parameters and Gut Bacterial Diversity. Front. Microbiol. 2021, 12, 623951. [Google Scholar] [CrossRef]
- Abriouel, H.; Casado Muñoz, M.D.C.; Lavilla Lerma, L.; Pérez Montoro, B.; Bockelmann, W.; Pichner, R.; Kabisch, J.; Cho, G.S.; Franz, C.M.A.P.; Gálvez, A.; et al. New insights in antibiotic resistance of Lactobacillus species from fermented foods. Food Res. Int. 2015, 78, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Vyas, V.; Mian, S.; Paolino, K.; Siddique, Z. Lactobacillus masticator abscess after probiotics consumption. Bayl. Univ. Med. Cent. 2020, 34, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Wasan, A.; Sharma, R.K. Recent developments in probiotics: An emphasis on Bifidobacterium. Food Biosci. 2021, 41, 100993. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Martins, F.C.O.L.; Sentanin, M.A.; Souza, D. Analytical methods in food additives determination: Compounds with functional applications. Food Chem. 2019, 272, 732–750. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, W.V. Realidades e Perspectivas em Ciência dos Alimentos Nova Xavantina, 2020. Available online: https://www.researchgate.net/publication/348310105_Realidades_e_Perspectivas_em_Ciencia_dos_Alimentos (accessed on 12 November 2023).
- Zang, E.; Jiang, L.; Cui, H.; Li, X.; Yan, Y.; Liu, Q.; Chen, Z.; Li, M. Only plant-based food additives: An overview on application, safety, and key challenges in the food industry. Food Rev. Int. 2022, 39, 5132–5163. [Google Scholar] [CrossRef]
- Shazly, A.B.; Fouad, M.T.; Elaaser, M.; Sayed, R.S.; Abd El-Aziz, M. Probiotic coffee ice cream as an innovative functional dairy food. J. Food Process. Preserv. 2022, 46, e17253. [Google Scholar] [CrossRef]
- Sales, A.L.; Paula, J.; Silva, C.M.; Cruz, A.; Miguel, M.A.L.; Farah, A. Effects of regular and decaffeinated roasted coffee (Coffea arabica and Coffea canephora) extracts and bioactive compounds on in vitro probiotic bacterial growth. Food Funct. 2020, 11, 1410–1424. [Google Scholar] [CrossRef]
- Muttaqin, Z.; Hadi, L.; Maghfirah, Z. Efficacy of Robusta Coffee Bean Extract (Coffea robusta) Against Bacterial Growth of Staphylococcus aureus. Biosci. Med. J. Biomed. Transl. Res. 2022, 6, 1675–1679. [Google Scholar] [CrossRef]
- Yangilar, F.; Yildiz, P.O. Effects of using combined essential oils on quality parameters of bio-yogurt. J. Food Process. Preserv. 2018, 42, 133–142. [Google Scholar] [CrossRef]
- Benguedouar, K.; Betina, S.B.; Erenler, R.; Genç, N.; Gok, M.; Sebti, M.; Madi, N.; Mekdade, L.; Gali, L.; Barkat, M. Evaluation of the antioxidant properties and total phenolic content of a dairy product (yogurt) supplemented with Thymus willdenowii essential oil from Algeria. J. Food Meas. Charact. 2022, 16, 3568–3577. [Google Scholar] [CrossRef]
- Canci, L.A.; De Toledo Benassi, M.; Canan, C.; Kalschne, D.L.; Colla, E. Antimicrobial potential of aqueous coffee extracts against pathogens and Lactobacillus species: A food matrix application. Food Biosci. 2022, 47, 101756. [Google Scholar] [CrossRef]
- Alfadhly, N.K.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and technological advancements in the possible food applications of Spirulina and their health benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef] [PubMed]
- Elfar, O.A.; Billa, N.; Lim, H.R.; Chew, K.W.; Cheah, W.Y.; Munawaroh, H.S.H.; Balakrishnan, D.; Show, P.L. Advances in delivery methods of Arthrospira platensis (Spirulina) for enhanced therapeutic outcomes. Bioengineered 2022, 13, 14681–14718. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, L.; Hosakawa, M.; Miyashita, K. Spirulina lipids alleviate oxidative stress and inflammation in mice fed a high-fat and high-sucrose diet. Mar. Drugs 2020, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Nakayama, S.M.M.; Kataba, A.; Yohannes, Y.B.; Ikenaka, Y.; Ishizuka, M. Evaluation of the ameliorative effect of Spirulina (Arthrospira platensis) supplementation on parameters relating to lead poisoning and obesity in C57BL/6J mice. J. Funct. Foods 2021, 77, 104344. [Google Scholar] [CrossRef]
- Montalvo, G.E.B.; Vandenberghe, L.P.D.S.; Soccol, V.T.; Carvalho, J.C.D.; Soccol, C.R. The antihypertensive, antimicrobial and anticancer peptides from Arthrospira with therapeutic potential: A mini review. Curr. Mol. Med. 2020, 20, 593–606. [Google Scholar] [CrossRef] [PubMed]
- El Baky, H.H.A.; El Baroty, G.S.; Mostafa, E.M. Optimization growth of Spirulina (Arthrospira) platensis in photobioreactor under varied nitrogen concentration for maximized biomass, carotenoids and lipid contents. Recent Pat. Food Nutr. Agric. 2020, 11, 40–48. [Google Scholar] [CrossRef]
- Silva, S.C.; Fernandes, I.P.; Barros, L.; Fernandes, A.; Alves, M.J.; Calhelha, R.C.; Pereira, C.; Barreira, J.C.M.; Manrique, Y.; Colla, E.; et al. Spray-dried Spirulina platensis as an effective ingredient to improve yogurt formulations: Testing different encapsulating solutions. J. Funct. Foods 2019, 60, 103427. [Google Scholar] [CrossRef]
- Patel, P.; Jethani, H.; Radha, C.; Vijayendra, S.V.N.; Mudliar, S.N.; Sarada, R.; Chauhan, V.S. Development of a carotenoid enriched probiotic yogurt from fresh biomass of Spirulina and its characterization. J. Food Sci. Technol. 2019, 56, 3721–3731. [Google Scholar] [CrossRef]
- Martin, M.A.; Ramos, S.; Mateos, R.; Granado Serrano, A.B.; Izquierdo-Pulido, M.; Bravo, L.; Goya, L. Protection of human HepG2 cells against oxidative stress by cocoa phenolic extract. J. Agric. Food Chem. 2008, 56, 7765–7772. [Google Scholar] [CrossRef] [PubMed]
- Hii, C.; Law, C.; Sharif, S.; Jati, M.; Cloke, M. Polyphenols in cocoa (Theobroma cacao L.). Asian J. Food Agroind. 2009, 2, 702–722. [Google Scholar]
- Carballeda Sangiao, N.; Chamorro, S.; de Pascual-Teresa, S.; Goya, L. Aqueous extract of cocoa phenolic compounds protects differentiated neuroblastoma SH-SY5Y cells from oxidative stress. Biomolecules 2021, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
- De La Luz Cádiz-Gurrea, M.; Fernández De Las Nieves, I.; Saez, L.M.A.; Fernández-Arroyo, S.; Legeai-Mallet, L.; Bouaziz, M.; Segura-Carretero, A. Bioactive Compounds from Theobroma cacao: Effect of Isolation and Safety Evaluation. Plant Foods Hum. Nutr. 2019, 74, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.D.; Ranadheera, S.; Fang, Z.; Ajlouni, S. Impact of encapsulating probiotics with cocoa powder on the viability of probiotics during chocolate processing, storage, and in vitro gastrointestinal digestion. J. Food Sci. 2021, 86, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.S.; Hussain, M.B.; Sultan, M.T.; Arshad, M.S.; Waheed, M.; Shariati, M.A.; Plygun, S.; Hashempur, M.H. Biochemistry, Safety, Pharmacological Activities, and Clinical Applications of Turmeric: A Mechanistic Review. Evid. Based Complement. Altern. Med. 2020, 10, 7656919. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K. Turmeric: Potential health benefits. Nutr. Today 2020, 55, 45–56. [Google Scholar] [CrossRef]
- Martina, E.C.; Oludayo, A.K.; Linda, N.C.; Chinasa, O.P.; Ambrose, O.C.; Muoneme, O.T. Effect of the incorporation of graded levels of turmeric (Curcuma longa) on different qualities of stirred yoghurt. Afr. J. Food Sci. 2020, 14, 71–85. [Google Scholar]
- Popescu, L.; Ghendov-Moșanu, A.; Baerle, A.; Savcenco, A.; Tatarov, P. Color stability of yogurt with natural yellow food dye from safflower (carthamus tinctorius L.). J. Eng. Sci. 2022, 29, 142–150. [Google Scholar] [CrossRef]
- Ghaffari, S.; Roshanravan, N. Saffron: An updated review on biological properties with special focus on cardiovascular effects. Biomed. Pharmacother 2019, 109, 21–27. [Google Scholar] [CrossRef] [PubMed]
- El Khoudri, M.; Ouahhoud, S.; Lahmass, M.; Khoulati, A.; Benyoussef, S.; Mamri, S.; Saalaoui, E. Biological effects and pharmacological activities of saffron of Crocus sativus. Arab. J. Med. Aromat. Plants 2021, 7, 254–268. [Google Scholar] [CrossRef]
- Roshanravan, N.; Ghaffari, S. The therapeutic potential of Crocus sativus Linn.: A comprehensive narrative review of clinical trials. Phytother Res. 2022, 36, 98–111. [Google Scholar] [CrossRef] [PubMed]
- El Midaoui, A.; Ghzaiel, I.; Vervandier-Fasseur, D.; Ksila, M.; Zarrouk, A.; Nury, T.; Lizard, G. Saffron (Crocus sativus L.): A source of nutrients for health and for the treatment of neuropsychiatric and age-related diseases. Nutrients 2022, 14, 597. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, R.; Gentile, C.; Bonanno, A.; Vintaloro, L.; Perrone, A.; Mazza, F.; Barbaccia, P.; Settanni, L.; Di Grigoli, A. Effect of Saffron Addition on the Microbiological, Physicochemical, Antioxidant and Sensory Characteristics of Yoghurt. Int. J. Dairy Technol. 2019, 72, 208–217. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Valero-Cases, E.; Julián Pastor, J.; Frutos, M.J. Microencapsulated Saffron Floral Waste Extracts as Functional Ingredients for Antioxidant Fortification of Yogurt: Stability during the Storage. Food Sci. Technol. 2023, 184, 114976. [Google Scholar] [CrossRef]
- Dabbagh Moghaddam, A.; Garavand, F.; Razavi, S.H.; Talatappe, H.D. Production of Saffron-based Probiotic Beverage by Lactic Acid Bacteria. J. Food Meas. Charact. 2018, 12, 2708–2717. [Google Scholar] [CrossRef]
- Camandola, S.; Plick, N.; Mattson, M.P. Impact of Coffee and Cacao Purine Metabolites on Neuroplasticity and Neurodegenerative Disease. Neurochem. Res. 2019, 44, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Pinsuwan, A.; Suwonsichon, S.; Chompreeda, P.; Prinyawiwatkul, W. Sensory drivers of consumer acceptance, purchase intent and emotions toward brewed black coffee. Foods 2022, 11, 180. [Google Scholar] [CrossRef]
- Jean-Marie, E.; Bereau, D.; Robinson, J.C. Benefits of polyphenols and methylxanthines from cocoa beans on dietary metabolic disorders. Foods 2021, 10, 2049. [Google Scholar] [CrossRef]
- Jean-Marie, E.; Jiang, W.; Bereau, D.; Robinson, J.C. Theobroma cacao and Theobroma grandiflorum: Botany, Composition and Pharmacological Activities of Pods and Seeds. Foods 2022, 11, 3966. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, N.; Samarghandian, S.; Hashemy, S.I. Immunomodulatory, anti-inflammatory, and antioxidant effects of curcumin. J. Herbmed. Pharmacol. 2018, 7, 211–219. [Google Scholar] [CrossRef]
- Azami, S.; Shahriari, Z.; Asgharzade, S.; Farkhondeh, T.; Sadeghi, M.; Ahmadi, F.; Forouzanfar, F. Therapeutic potential of saffron (Crocus sativus L.) in ischemia stroke. Evid. Based Complement. Altern. Med. 2021, 2021, 6643950. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Thakur, R.; Kumar, R. Saffron (Crocus sativus L.): Gold of the spices-A comprehensive review. Hortic. Environ. Biotechnol. 2021, 62, 661–677. [Google Scholar] [CrossRef]
- Kumar, T.M.; Padmavathi, M.N.T. Development and evaluation of spirulina ragi biscuits. Int. J. Cardiovasc. Sci. 2020, 8, 208–210. [Google Scholar] [CrossRef]
- Jahan, F.; Mishra, S. Preparation and Quality Evaluation of Iron Binding Protein Augumented Dhokla Using Spirulina and Other Natural Ingredients. Asian J. Agric. Res. 2021, 8, 48–56. [Google Scholar] [CrossRef]
- Koli, D.K.; Rudra, S.G.; Bhowmik, A.; Pabbi, S. Nutritional, Functional, Textural and Sensory Evaluation of Spirulina Enriched Green Pasta: A Potential Dietary and Health Supplement. Foods 2022, 11, 979. [Google Scholar] [CrossRef]
- Yamgar, P.V.; Dhamak, M. Therapeutics role of spirulina platensis in disease prevention and treatment. Int. J. Compr. Adv. Pharmacol. 2022, 7, 30–39. [Google Scholar] [CrossRef]
- Atwaa, E.S.H.; Shahein, M.R.; El-Sattar, E.S.A.; Hijazy, H.H.A.; Albrakati, A.; Elmahallawy, E.K. Bioactivity, physicochemical and sensory properties of probiotic yoghurt made from whole milk powder reconstituted in aqueous fennel extract. Fermentation 2022, 8, 52. [Google Scholar] [CrossRef]
- Kim, E.D.; Lee, H.S.; Kim, K.T.; Paik, H.D. Antioxidant and Angiotensin-Converting Enzyme (ACE) Inhibitory activities of yogurt supplemented with Lactiplantibacillus plantarum NK181 and Lactobacillus delbrueckii KU200171 and sensory evaluation. Foods 2021, 10, 2324. [Google Scholar] [CrossRef]
- Akan, E.; Yerlikaya, O.; Bayram, O.Y.; Kinik, O. Viability of Probiotics, Rheological and the Sensorial Properties of Probiotic Yogurts Fortified with Aqueous Extracts of Some Plants. An. Acad. Bras. Ciênc. 2022, 94, e20211274. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Avilés, E.; García-Pérez, M.E.; Garnica-Romo, M.G.; de Dios Figueroa-Cárdenas, J.; Paciulli, M.; Martinez-Flores, H.E. Chemical composition, physicochemical evaluation and sensory analysis of yogurt added with extract of polyphenolic compounds from Quercus crassifolia oak bark. Funct. Foods Health Dis. 2022, 12, 502–517. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Q. EPS-Producing Lactobacillus plantarum MC5 as a compound starter improves rheology, texture, and antioxidant activity of yogurt during storage. Foods 2022, 11, 1660. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.I.; Dey, M.; Alzubaidi, A.K.; Alneamah, S.J.A.; Altemimi, A.B.; Pratap-Singh, A. Effect of rosemary (Rosmarinus officinalis L.) supplementation on probiotic yoghurt: Physicochemical properties, microbial content, and sensory attributes. Foods 2021, 10, 2393. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, R.M.; Ariaii, P.; Ahmady, M. The effect of microencapsulated extract of pennyroyal (Mentha pulegium. L) on the physicochemical, sensory, and viability of probiotic bacteria in yogurt. J. Food Meas. Charact. 2021, 15, 2625–2636. [Google Scholar] [CrossRef]
- Gouda, A.S.; Adbelruhman, F.G.; Alenezi, H.S.; Mégarbane, B. Theoretical benefits of yogurt-derived bioactive peptides and probiotics in COVID-19 patients-A narrative review and hypotheses. Saudi J. Biol. Sci. 2021, 28, 5897–5905. [Google Scholar] [CrossRef] [PubMed]
- Mahfudh, N.; Hadi, A.; Solechan, R.A.Z. Immunomodulatory activity of yogurt fortified with roselle (Hibiscus sabdariffa L.) extract. Int. Food Res. J. 2021, 28, 2. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Alqah, H.A.; Saleh, A.; Al-Juhaimi, F.Y.; Babiker, E.E.; Ghafoor, K.; Hassan, A.B.; Osman, M.A.; Fickak, A. Physicochemical quality attributes and antioxidant properties of set-type yogurt fortified with argel (Solenostemma argel Hayne) leaf extract. LWT 2021, 137, 110389. [Google Scholar] [CrossRef]
- Gris, C.C.T.; Frota, E.G.; Guarienti, C.; Vargas, B.K.; Gutkoski, J.P.; Biduski, B.; Bertolin, T.E. In vitro digestibility and stability of encapsulated yerba mate extract and its impact on yogurt properties. J. Food Meas. Charact. 2021, 15, 2000–2009. [Google Scholar] [CrossRef]
- Song, M.W.; Park, J.Y.; Lee, H.S.; Kim, K.T.; Paik, H.D. Co-fermentation by Lactobacillus brevis B7 improves the antioxidant and immunomodulatory activities of hydroponic ginseng-fortified yogurt. Antioxidants 2021, 10, 1447. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Functional and healthy yogurts fortified with probiotics and fruit peel powders. Fermentation 2022, 8, 469. [Google Scholar] [CrossRef]
- Ziarno, M.; Kozłowska, M.; Ścibisz, I.; Kowalczyk, M.; Pawelec, S.; Stochmal, A.; Szleszyński, B. The Effect of Selected Herbal Extracts on Lactic Acid Bacteria Activity. Appl. Sci. 2021, 11, 3898. [Google Scholar] [CrossRef]
- Morales-de la Peña, M.; Miranda-Mejía, G.A.; Martín-Belloso, O. Recent Trends in Fermented Beverages Processing: The Use of Emerging Technologies. Beverages 2023, 9, 51. [Google Scholar] [CrossRef]
- Afzaal, M.; Khan, A.U.; Saeed, F.; Ahmed, A.; Ahmad, M.H.; Maan, A.A.; Tufail, T.; Anjum, F.M.; Hussain, S. Functional exploration of free and encapsulated probiotic bacteria in yogurt and simulated gastrointestinal conditions. Food Sci. Nutr. 2019, 7, 3931–3940. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Probiotic viability and physico-chemical and sensory properties of plain and stirred fruit yogurts made from goat’s milk. Food Chem. 2012, 135, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Mortazavian, A.M.; Ghorbanipour, S.; Mohammadifar, M.A.; Mohammadi, M. Biochemical properties and viable probiotic population of yogurt at different bacterial inoculation rates and incubation temperatures. Phil. Agric. Sci. 2011, 94, 111–116. [Google Scholar]
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Darani, K.K. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- Akalın, A.S.; Unal, G.; Dinkci, N.; Hayaloglu, A.A. Microstructural, textural, and sensory characteristics of probiotic yogurts fortified with sodium calcium caseinate or whey protein concentrate. J. Dairy Sci. 2012, 95, 3617–3628. [Google Scholar] [CrossRef]
- De Simone, C. The unregulated probiotic market. Clin. Gastroenterol. Hepatol. 2019, 17, 809–817. [Google Scholar] [CrossRef]
- Saarela, M.H. Safety aspects of next generation probiotics. Curr. Opin. Food Sci. 2018, 30, 8–13. [Google Scholar] [CrossRef]
- Turgut, T.; Cakmakci, S. Probiotic strawberry yogurts: Microbiological, chemical and sensory properties. Probiotics Antimicrob. Proteins 2018, 10, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probióticos na indústria de laticínios—Avanços e oportunidades. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982. [Google Scholar] [CrossRef] [PubMed]
- Ilango, S.; Usha, A. Microrganismos probióticos de alimentos fermentados tradicionais não lácteos. Trends. Food. Sci. Technol. 2021, 118, 617–638. [Google Scholar] [CrossRef]
- Parker, M.; Zobrist, S.; Donahue, C.; Edick, C.; Mansen, K.; Nadjari, M.H.Z.; Heerikhuisen, M.; Sybesma, W.; Molenaar, D.; Diallo, A.M.; et al. Naturally Fermented Milk From Northern Senegal: Bacterial Community Composition and Probiotic Enrichment With Lactobacillus rhamnosus. Front. Microbiol. 2018, 9, 2218. [Google Scholar] [CrossRef] [PubMed]
- El Bouchikhi, S.; Pagès, P.; El Alaoui, Y.; Ibrahimi, A.; Bensouda, Y. Syneresis investigations of lacto-fermented sodium caseinate in a mixed model system. BMC Biotechnol. 2019, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, L.; Zhang, J.; Wang, L.; Dong, M.; McClements, D.J.; Wan, F.; Shen, P.; Li, Q. Reinforcing Alginate Matrixes by Tea Polysaccharide Conjugates or Their Stabilized Nanoemulsion for Probiotics Encapsulation: Characterization, Survival after Gastrointestinal Digestion and Ambient Storage. Int. J. Biol. Macromol. 2023, 253, 126828. [Google Scholar] [CrossRef] [PubMed]
- Vimon, S.; Kertsomboon, T.; Chirachanchai, S.; Kris Angkanaporn, K.; Nuengjamnong, C. Matrices-charges of Agar-alginate Crosslinked Microcapsules via O/w Microemulsion: A Non-spore Forming Probiotic Bacteria Encapsulation System for Extensive Viability. Carbohydr. Polym. 2023, 321, 121302. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Muñoz, Y.V.; Santagapita, P.R.; Quintanilla-Carvajal, M.X. Probiotic Encapsulation: Bead Design Improves Bacterial Performance during In Vitro Digestion. Polymers 2023, 15, 4296. [Google Scholar] [CrossRef]
- Bisanz, J.E.; Macklaim, J.M.; Gloor, G.B.; Reid, G. Bacterial metatranscriptome analysis of a probiotic yoghurt using an RNA-Seq approach. Int. Dairy J. 2014, 39, 284–292. [Google Scholar] [CrossRef]
- Terpou, A.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A.; Nigam, P. Enhanced probiotic viability and aromatic profile of yogurts producedusing wheat bran (Triticum aestivum) as cell immobilization carrier. Process Biochem. 2017, 55, 1–10. [Google Scholar] [CrossRef]
- Marsh, A.J.; Colin, H.; Paul, R.P.; Cotter, P.D. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef]
- Kamal, R.M.; Alnakip, M.E.; El Aal, S.F.A.; Bayoumi, M.A. Bio-controlling capability of probiotic strain Lactobacillus rhamnosus against some common foodborne pathogens in yoghurt. Int. Dairy J. 2018, 85, 1–7. [Google Scholar] [CrossRef]
- Zhang, T.; Jeong, C.H.; Cheng, W.N.; Bae, H.; Seo, H.G.; Petriello, M.C.; Han, C.G. Moringa extract enhances the fermentative, textural, and bioactive properties of yoghurt. LWT 2019, 101, 276–284. [Google Scholar] [CrossRef]
- Aryana, K.J.; Olson, D.W.A. 100-year review: Yoghurt and other cultured dairy products. J. Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef] [PubMed]
- Franco, B.D.G.M.; Landgraf, M. Microbiologia dos Alimentos, 2nd ed.; Atheneu: Rio de Janeiro, Brazil, 2023; p. 292. [Google Scholar]
- Hekmat, S.; Soltani, H.; Rei, G. Growth and survival of Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR-1 in yogurt for use as a functional food. Innov. Food Sci. Emerg. Technol. 2009, 10, 293–296. [Google Scholar] [CrossRef]
- Abd El-Gawad, I.A.; El-Sayed, E.M.; El-Zeini, H.M.; Hafez, S.A.; Saleh, F.A. Antibacterial activity of probiotic yoghurt and soy-yoghurt against Escherichia coli and Staphylococcus aureus. J. Nutr. Food Sci. 2014, 4, 1000303. [Google Scholar]
- Matejčeková, Z.; Spodniaková, S.; Koňuchová, M.; Liptáková, D.; Valík, L. In Vitro Growth Competition of Lactobacillus plantarum HM1 with Pathogenic and Food Spoilage Microorganisms. J. Food Nutr. Res. 2019, 58, 236–244. [Google Scholar]
- Barba, F.J.; Sant’ana, A.S.; Orlien, V.; Koubaa, M.; Barba, F.; Sant’ana, A. Innovative Technologies for Food Preservation: Inactivation of Spoilage and Pathogenic Microorganisms; Academic Press: Cambridge, UK, 2017. [Google Scholar]
- Sarwar, A.; Al-Dalali, S.; Aziz, T.; Yang, Z.; Ud Din, J.; Khan, A.A.; Daudzai, Z.; Syed, Q.; Nelofer, R.; Qazi, N.U.; et al. Effect of chilled storage on antioxidant capacities and volatile flavors of synbiotic yogurt made with probiotic yeast Saccharomyces boulardii CNCM I-745 in combination with inulin. J. Fungi 2022, 8, 713. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Behbahani, M. Enhancement of probiotics viability and lactic acid production in yogurts treated with Prangos ferulaceae and Carum copticum plant extracts. Biocatal. Agric. Biotechnol. 2021, 35, 102084. [Google Scholar] [CrossRef]
- Ghafoor, K.; Sarker, M.Z.I.; Al-Juhaimi, F.Y.; Mohamed Ahmed, I.A.; Babiker, E.E.; Alkaltham, M.S.; Almubarak, A.K. Bioactive Compounds Extracted from Saudi Dates Using Green Methods and Utilization of These Extracts in Functional Yogurt. Foods 2023, 12, 847. [Google Scholar] [CrossRef]
- Inocente-Camones, M.A.; Arias-Arroyo, G.C.; Mauricio-Alza, S.M.; Bravo-Araujo, G.T.; Capcha-Siccha, M.F.; Cabanillas-Alvitrez, E. Polyphenols, carotenoids and flavonoids in an antioxidant probiotic yogurt made with tumbo pulp (Passiflora tripartita Kunth). Braz. J. Food Technol. 2022, 25, e2021175. [Google Scholar] [CrossRef]
- Safdari, Y.; Vazifedoost, M.; Didar, Z.; Hajirostamloo, B. The Effect of Banana Fiber and Banana Peel Fiber on the Chemical and Rheological Properties of Symbiotic Yogurt Made from Camel Milk. Int. J Food Sci. 2021, 15, 5230882. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, M.; Drunkler, D.A.; Colla, E. Probiotic Functional Yogurt: Challenges and Opportunities. Fermentation 2024, 10, 6. https://doi.org/10.3390/fermentation10010006
de Souza M, Drunkler DA, Colla E. Probiotic Functional Yogurt: Challenges and Opportunities. Fermentation. 2024; 10(1):6. https://doi.org/10.3390/fermentation10010006
Chicago/Turabian Stylede Souza, Marinêz, Deisy Alessandra Drunkler, and Eliane Colla. 2024. "Probiotic Functional Yogurt: Challenges and Opportunities" Fermentation 10, no. 1: 6. https://doi.org/10.3390/fermentation10010006
APA Stylede Souza, M., Drunkler, D. A., & Colla, E. (2024). Probiotic Functional Yogurt: Challenges and Opportunities. Fermentation, 10(1), 6. https://doi.org/10.3390/fermentation10010006