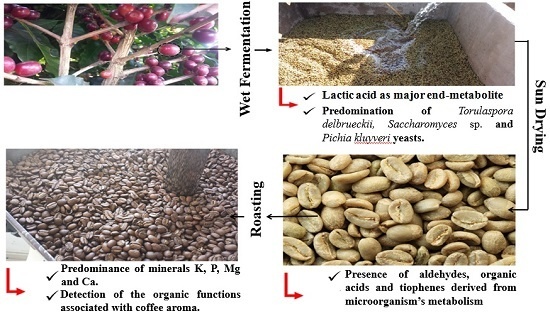
Yeast Diversity and Physicochemical Characteristics Associated with Coffee Bean Fermentation from the Brazilian Cerrado Mineiro Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spontaneous Coffee Bean Fermentation and Sampling
2.2. Yeast Isolation
2.3. Identification of Yeast Isolates
2.4. High Performance Liquid Chromatography (HPLC) Analysis of Fermenting Coffee Beans Samples
2.5. Physicochemical Characterization of Fermented and Roasted Coffee Beans
2.5.1. Volatile Organic Compounds Determination by Gas Chromatography Coupled to Mass Spectrophotometry (GC-MS)
2.5.2. Mineral Analysis
2.5.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.5.4. Thermal Stability
3. Results and Discussion
3.1. Microbiological and Chemical Characterization of Coffee Bean Fermentation
3.2. Yeast Identification
3.3. Volatile Organic Compounds Determination of Fermented Cocoa Beans by Gas Chromatography Coupled to Mass Spectrophotometry (GC-MS)
3.4. Physicochemical Characterization of Coffee Beans
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pereira, G.V.M.; Soccol, V.T.; Pandey, A.; Medeiros, A.B.P.; Lara, J.M.R.A.; Gollo, A.L.; Soccol, C.R. Isolation, selection and evaluation of yeasts for use in fermentation of coffee beans by the wet process. Int. J. Food Microbiol. 2014, 188, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, S.R.; Silva, C.R.; Miguel, M.G.P.C.; Cordeiro, C.S.; Pinheiro, A.C.M.; Duarte, W.F.; Schwan, R.F. Improvement of coffee beverage quality by using selected yeasts strains during the fermentation in dry process. Food Res. Int. 2014, 61, 183–195. [Google Scholar] [CrossRef]
- Evangelista, S.R.; Miguel, M.G.C.P.; Silva, C.P.; Pinheiro, A.C.M.; Schwan, R.F. Microbiological diversity associated with the spontaneous wet method of coffee fermentation. Int. J. Food Microbiol. 2015, 210, 102–112. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; Neto, E.; Soccol, V.T.; Medeiros, A.B.P.; Woiciechowski, A.L.; Soccol, C.R. Conducting starter culture-controlled fermentations of coffee beans during on-farm wet processing: Growth, metabolic analyses and sensorial effects. Food Res. Int. 2015, 75, 348–356. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; Carvalho-Neto, D.P.; Medeiros, A.B.P.; Soccol, V.T.; Neto, E.; Woiciechowski, A.L.; Soccol, C.R. Potential of lactic acid bacteria to improve the fermentation of coffee during on-farm processing. Int. J. Food Sci. Technol. 2016, 51, 1689–1695. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; Soccol, V.T.; Brar, S.K.; Neto, E.; Soccol, C.R. Microbial ecology and starter culture technology in coffee processing. Crit. Rev. Food Sci. 2015. [Google Scholar] [CrossRef]
- Wootton, A.E. The fermentation of coffee. Kenya Coffee 1963, 28, 239–249. [Google Scholar]
- Sivetz, M. Coffee Processing Technology; AVI: Westport, CT, USA, 1963; Volume 2. [Google Scholar]
- Masoud, W.; Cesar, L.B.; Jespersen, L.; Jakobsen, M. Yeast involved in fermentation of Coffea arabica in East Africa determined by genotyping and by direct denaturating gradient gel electrophoresis. Yeast 2004, 21, 549–556. [Google Scholar] [CrossRef]
- Silva, C.F.; Batistam, L.R.; Abreu, L.M.; Dias, E.S.; Schwan, R.F. Succession of bacterial and fungal communities during natural coffee (Coffea arabica) fermentation. Food Microbiol. 2008, 25, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Vilela, D.M.; Pereira, G.V.M.; Silva, C.F.; Batista, L.R.; Schwan, R.F. Molecular ecology and polyphasic characterization of the microbiota associated with semi-dry processed coffee (Coffea arabica L.). Food Microbiol. 2010, 27, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- The Association of Brazilian Coffee Producing Regions. Available online: http://www.abopcafe.com.br/cerrado-mineiro/ (accessed on 19 January 2017).
- Avallone, S.; Guyot, B.; Brillouet, J.M.; Olguin, E.; Guiraud, J.P. Microbiological and biochemical study of coffee fermentation. Curr. Microbiol. 2001, 42, 252–256. [Google Scholar] [CrossRef]
- Velmourougane, K. Impact of natural fermentation on physicochemical, microbiological and cup quality characteristics of Arabica and Robusta coffee. Proc. Natl. Acad. Sci. USA 2013, 83, 233–239. [Google Scholar] [CrossRef]
- Lopez, C.I.; Bautista, E.; Moreno, E.; Dentan, E. Factors related to the formation of “overfermented coffee beans” during the wet processing method and storage of coffee. In Proceedings of the 13th International Scientific Colloquium on Coffee, Paipa, Colombia, 21–25 August 1989.
- Silva, C.F.; Vilela, D.M.; de Souza, C.C.; Duarte, W.F.; Dias, D.R.; Schwan, R.F. Evaluation of a potential starter culture for enhance quality of coffee fermentation. World J. Microbiol. Biotechnol. 2013, 29, 235–247. [Google Scholar] [CrossRef]
- Blomberg, A.; Alder, L. Physiology of osmotolerance in fungi. Adv. Microbiol. Phys. 1992, 33, 145–212. [Google Scholar]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. Microbial production of organic acids: Expanding the markets. Trends Biotechnol. 2008, 26, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; Belloch, C.; Vallés, S.; Manzanares, P. Monitoring a mixed starter of Hanseniaspora vineae-Saccharomyces cerevisiae in natural must: Impact on 2-phenylethyl acetate production. Int. J. Food Microbiol. 2011, 151, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Lleixà, J.; Martín, V.; Portillo, M.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of fermentation and wines produced by inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [PubMed]
- Damiani, P.; Gobbetti, M.; Cossignani, L.; Corsetti, A.; Simonetti, M.S.; Rossi, J. The sourdrough microflora. Characterization of hetero- and homofermentative lactic acid bacteria, yeasts and their interactions on the basis of the volatile compounds produced. LWT Food Sci. Technol. 1996, 29, 63–70. [Google Scholar] [CrossRef]
- Owusu, M.; Petersen, M.A.; Heimdal, H. Effect of fermentation method, roasting and conching conditions on the aroma volatiles of dark chocolate. J. Food Process. Preserv. 2012, 36, 446–456. [Google Scholar] [CrossRef]
- Martín, M.J.; Pablosm, F.; González, A.G. Characterization of green coffee varieties according to their metal content. Anal. Chim. Acta 1998, 358, 177–183. [Google Scholar]
- Franková, A.; Drábekm, O.; Havlík, J.; Száková, J.; Vanek, A. The effect of beverage preparation method on aluminum content in coffee infusions. J. Inorg. Biochem. 2009, 103, 1480–1485. [Google Scholar]
- Sinija, V.; Mishra, H. FT-NIR spectroscopy for caffeine estimation in instant green tea powder and granules. LWT Food Sci. Technol. 2009, 42, 998–1002. [Google Scholar] [CrossRef]
- Paradkar, M.M.; Irudayaraj, J. A rapid FTIR spectroscopic method for estimation of caffeine in soft drinks and total methylxanthines in tea and coffee. J. Food Sci. 2002, 67, 2507–2511. [Google Scholar] [CrossRef]
- Giovenzana, V.; Beghi, R.; Guidetti, R. Rapid evaluation of craft beer quality during fermentation process by Vis/NIR spectroscopy. J. Food Eng. 2014, 142, 80–86. [Google Scholar] [CrossRef]
- Krähmer, A.; Engel, A.; Kadow, D.; Ali, N.; Umaharan, P.; Kroh, L.W.; Schulz, H. Fast and neat—Determination of biochemical quality parameters in cocoa using near infrared spectroscopy. Food Chem. 2015, 181, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Lyman, D.J.; Benck, R.; Dell, S.; Merle, S.; Murray-Wijelath, J. FTIR-ATR analysis of brewed coffee: Effect of roasting conditions. J. Agric. Food Chem. 2003, 51, 3268–3272. [Google Scholar] [CrossRef]
- Yeretzian, C.; Jordan, A.; Badoud, R.; Lindinger, W. From the green bean to cup of coffee: Investigating coffee roasting by on-line monitoring of volatiles. Eur. Food Res. Technol. 2002, 214, 92–104. [Google Scholar] [CrossRef]
- Jakab, E.; Faix, O.; Till, F. Thermal decomposition of milled wood lignins studied by thermogravimetry/mass spectrometry. J. Anal. Appl. Pyrolysis 1997, 40–41, 171–186. [Google Scholar] [CrossRef]
- Ouajai, S.; Shanks, R.A. Composition, structure and thermal degradation of hemp cellulose after chemical treatments. Polym. Degrad. Stabil. 2005, 89, 237–335. [Google Scholar] [CrossRef]





| Organic Functional Group | Volatile Organic Compound |
|---|---|
| Aldehydes (2) | Nonanal Heptanal |
| Alcohol (6) | 5-tert-Butyl-1,3-cyclohexanediol 2-Propyl-1-pentanol 4-Ethyl-1-octyn-3-ol 2-ethyl-1-Decanol |
| Carboxylic acid (4) | 13-(Benzyloxy) tridecanoic acid Dodecanoic acid, 3-hydroxy- Methacrylic acid Acetic acid |
| Ester (1) | Heptyl valerate |
| Hydrocarbons (9) | Heptane, 2,2,3,5-tetramethyl-Pentane, 2,2,3,4-tetramethyl-Heptane, 2,2,6,6-tetramethyl-Hexane, 2,2,5-trimethyl-Eicosane, 3-methyl-Dodecane, 2,6,11-trimethyl-Heptane, 5-ethyl-2,2,3-trimethyl-Hexadecane 9-Octadecene, 1,1-dimethoxy- |
| Sulfur Compounds (1) | 2-Allyl-3-methyl-4-(phenylsulfanyl)-2,5-dihydrothiophene, 1,1-dioxide |
| Ketone (1) | p-Benzoquinone |
| Pyrazine (1) | 2-Isobutyl-3-methoxypyrazine |
| Minerals (mg·kg−1) | Trait | |
|---|---|---|
| Fermented Coffee | Roasted Coffee | |
| Al | 234.71 ± 29.22 a | 362.56 ± 106.12 b |
| Ba | 3.40 ± 0.49 c | 2.61 ± 0.09 c |
| B | 7.28 ± 0.30 c | 6.26 ± 0.21 c |
| Cd | ND c | ND c |
| Ca | 1360.19 ± 20.53 d | 1192.32 ± 0.88 e |
| Co | ND c | ND c |
| Cu | 16.51 ± 0.38 c | 17.57 ± 0.25 c |
| Fe | 33.75 ± 1.32 c | 33.12 ± .44 c |
| P | 1932.76 ± 43.56 f | 2110.71 ± 28.40 g |
| Li | ND c | ND c |
| Mg | 1554.31 ± 17.47 h | 1772.65 ± 24.87 i |
| Mn | 16.32 ± 0.01c | 17.43 ± 0.07 c |
| Mo | ND c | ND c |
| Ni | ND c | ND c |
| K | 12453.10 ± 8.35 j | 13117.50 ± 16.41 k |
| Se | ND c | ND c |
| Na | 350.565 ± 17.83 b | 380.99 ± 16.40 b |
| V | 0.86 ± 0.05 c | 0.47 ± 0.04 c |
| Zn | 10.15 ± 0.12 c | 9.78 ± 0.30 c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Carvalho Neto, D.P.; De Melo Pereira, G.V.; Tanobe, V.O.A.; Thomaz Soccol, V.; G. da Silva, B.J.; Rodrigues, C.; Soccol, C.R. Yeast Diversity and Physicochemical Characteristics Associated with Coffee Bean Fermentation from the Brazilian Cerrado Mineiro Region. Fermentation 2017, 3, 11. https://doi.org/10.3390/fermentation3010011
De Carvalho Neto DP, De Melo Pereira GV, Tanobe VOA, Thomaz Soccol V, G. da Silva BJ, Rodrigues C, Soccol CR. Yeast Diversity and Physicochemical Characteristics Associated with Coffee Bean Fermentation from the Brazilian Cerrado Mineiro Region. Fermentation. 2017; 3(1):11. https://doi.org/10.3390/fermentation3010011
Chicago/Turabian StyleDe Carvalho Neto, Dão Pedro, Gilberto Vinícius De Melo Pereira, Valcineide O. A. Tanobe, Vanete Thomaz Soccol, Bruno José G. da Silva, Cristine Rodrigues, and Carlos Ricardo Soccol. 2017. "Yeast Diversity and Physicochemical Characteristics Associated with Coffee Bean Fermentation from the Brazilian Cerrado Mineiro Region" Fermentation 3, no. 1: 11. https://doi.org/10.3390/fermentation3010011
APA StyleDe Carvalho Neto, D. P., De Melo Pereira, G. V., Tanobe, V. O. A., Thomaz Soccol, V., G. da Silva, B. J., Rodrigues, C., & Soccol, C. R. (2017). Yeast Diversity and Physicochemical Characteristics Associated with Coffee Bean Fermentation from the Brazilian Cerrado Mineiro Region. Fermentation, 3(1), 11. https://doi.org/10.3390/fermentation3010011