Laccases as a Potential Tool for the Efficient Conversion of Lignocellulosic Biomass: A Review
Abstract
:1. Introduction
2. Lignocellulosic Biomass Conversion: The Sugar Platform
3. Inhibitors and Lignin in Pretreated Materials
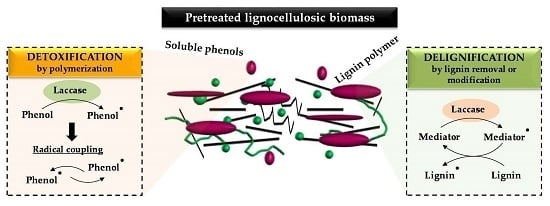
3.1. Detoxification of Pretreated Materials
3.2. Delignification of Pretreated Materials
4. Outline of Laccase Enzymes
Laccase-Mediator Systems (LMS)
5. Application of Laccases for Detoxification of Pretreated Materials
5.1. Detoxification Mechanism
5.2. Detoxification and Fermentation
5.3. Detoxification and Saccharification
5.4. Other Comments
6. Application of Laccases for Delignification of Pretreated Materials
6.1. Delignification by Laccase Alone
6.2. Delignification by Laccase-Mediator System (LMS)
6.3. Other Comments
7. Laccases for Detoxification and Delignification in a Lignocellulose-based Biorefinery
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moreno, A.D.; Alvira, P.; Ibarra, D.; Tomás-Pejó, E. Production of ethanol from lignocellulosic biomass. In Production of Chemicals from Sustainable Resources, Biofuels and Biorefineries 7; Fang, Z., Smith, R.L., Jr., Richard, L., Xinhua, Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Balan, V.; Chiaramonti, D.; Kumar, S. Review of US and EU initiatives toward development, demonstration, and commercialization of lignocellulosic biofuels. Biofuels Bioprod. Biorefin. 2013, 7, 732–759. [Google Scholar] [CrossRef]
- Ballesteros, M. Enzymatic hydrolysis of lignocellulosic biomass. In Bioalcohol Production. Biochemical Conversion of Lignocellulosic Biomass; Waldron, K., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 159–177. [Google Scholar]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanism of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M.; Gilkes, N.; Kadla, J.; Maximenko, V.; Kubo, S. Inhibition of cellulase, xylanase and β-glucosidase activities by sofwood lignin preparations. J. Biotechnol. 2006, 125, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Silvério da Silva, S.; Singh, O.V. Detoxification of lignocellulosic hydrolysates for improved bioethanol production. In Biofuel-Production-Recent Development Prospect; Aurelio, M., Bernardes, S., Eds.; InTechOpen: Rijeka, Croatia, 2011; pp. 225–246. [Google Scholar]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. A review of biological delignification and detoxification methods for lignocellulosic bioethanol production. Crit. Rev. Biotechnol. 2015, 35, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Parawira, W.; Tekere, M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef] [PubMed]
- E4tech; RE-CORD; WUR. From the Sugar Platform to Biofuels and Biochemicals. Final Report for the European Commission Directorate-General Energy, Contract No. ENER/C2/423-2012/SI2.673791: European Union. 2015. Available online: http://ibcarb.com/wp-content/uploads/EC-Sugar-Platform-final-report.pdf (accessed on 19 March 2017).
- Mosier, N.; Wyman, C.E.; Dale, B.D.; Elander, R.T.; Lee, Y.Y.; Holtzapple, M.; Ladisch, C.M. Features of promising technologies for pretreament of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Xie, D.; Gilkes, N.; Gregg, D.J.; Sadler, J.N. Strategies to enhance the enzymatic hydrolysis of pretreated softwood with high residual content. Appl Biochem. Biotechnol. 2005, 124, 1069–1079. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Mooney, C.; Saddler, J.N. Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol. Prog. 1999, 15, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.P.; Bura, R.; Mabee, W.E.; Berlin, A.; Pan, X.; Sadler, J.N. Substrate pretreatment: The key to effective enzymatic hydrolysis of lignocellulosics? Adv. Biochem. Eng. Biotechnol. 2007, 108, 67–83. [Google Scholar] [PubMed]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Mussato, S.I.; Dragone, G.M. Biomass pretreatment, biorefineries, and potential products for a bioeconomy development. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Biorefinery; Mussato, S.I., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 1–22. [Google Scholar]
- Alvira, P.; Ballesteros, M.; Negro, M.J. Progress on enzymatic saccharification technologies for biofuels production. In Biofuel Technologies: Recent Developments; Gupta, V.K., Tuohy, G., Eds.; Springer: Berlin, Germany, 2013; pp. 145–169. [Google Scholar]
- Jørgensen, H.; Krinstensen, J.B.; Felby, C. Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels Bioprod. Biorefin. 2007, 1, 119–134. [Google Scholar] [CrossRef]
- Martínez, A. How to break down crystalline cellulose. Science 2016, 352, 1050–1051. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.; Jørgensen, H.; Krogh, K.B.R.; Roca, C. Bioethanol production from lignocellulosic material. In Polysaccharides: Structural Diversity and Functional Versatility; Dumitriu, S., Ed.; Marcel Dekker: New York, NY, USA, 2005; pp. 957–993. [Google Scholar]
- Radecka, D.; Mukherjee, V.; Mateo, R.Q.; Stojiljkovic, M.; Foulquie-Moreno, M.R.; Thevelien, J.M. Looking beyond Saccharomyces: The potential of non-conventional yeast species for desirable traits in bioethanol fermentation. FEMS Yeast Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.F.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies form minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.J.; Karimi, K. Fermentation inhibitors in ethanol processes and different strategies to reduce their effects. In Biofuels: Alternative Feedstocks and Conversion Processes; Pandey, A., Larroche, C., Ricke, S.C., Dussap, C.G., Gnansounou, E., Eds.; Springer: Berlin, Germany, 2011; pp. 287–311. [Google Scholar]
- Panagiotou, G.; Olsson, L. Effect of compounds released during pretreatment of wheat straw on microbial growth and enzymatic hydrolysis rates. Biotechnol. Bioeng. 2006, 96, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Deactivation of cellulases by phenols. Enzyme Microb. Technol. 2011, 48, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.T.; Ruíz-Dueñas, J.; Martínez, M.J.; Del Río, J.C.; Gutiérrez, A. Enzymatic delignification of plant cell wall: From nature to mill. Curr. Opin. Biotechnol. 2009, 20, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Demedts, B.; Morrel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Rahikainen, J.L.; Martín-Sampedro, R.; Heikkinen, H.; Rovio, S.; Marjamaa, K.; Tamminen, T.; Rojas, O.J.; Kruus, K. Inhibitory effect of lignin during cellulose bioconversion: The effect of lignin chemistry on non-productive enzyme adsorption. Bioresour. Technol. 2013, 133, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.I.; Martín-Sampedro, R.; Fillat, U.; Oliva, J.M.; Negro, M.J.; Ballesteros, M.; Eugenio, M.E.; Ibarra, D. Evaluating lignin-rich residues from biochemical ethanol production of wheat straw and olive tree pruning by FTIR and 2D-NMR. Int. J. Polym. Sci. 2015. [Google Scholar] [CrossRef]
- Santos, J.I.; Fillat, U.; Martín-Sampedro, R.; Ballesteros, I.; Manzanares, P.; Ballesteros, M.; Eugenio, M.E.; Ibarra, D. Lignin-enriched fermentation residues from bioethanol production of fast-growing poplar and forage sorghum. Bioresources 2015, 10, 5215–5232. [Google Scholar] [CrossRef]
- Li, J.; Henriksson, G.; Gellerstedt, G. Lignin depolymerization/repolymerization and its critical role for delignification of aspen wood by steam-explosion. Bioresour. Technol. 2007, 98, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.; Cao, S.; Das, B.K.; Hu, F.; Pu, Y.; Ragauskas, A.J. Investigation of the fate of poplar lignin during autohydrolysis pretreatment to understand the biomass recalcitrance. RSC Adv. 2013, 3, 5305–5309. [Google Scholar] [CrossRef]
- Sewalt, V.J.H.; Glasser, W.G.; Beauchemin, K.A. Lignin impact on fiber degradation. 3. Reversal of inhibition of enzymatic hydrolysis by chemical modification of lignin and by additives. J. Agric. Food. Chem. 1997, 45, 1823–1828. [Google Scholar] [CrossRef]
- Helle, S.S.; Duff, S.J.; Cooper, D.G. Effect of surfactants on cellulose hydrolysis. Biotechnol. Bioeng. 1993, 42, 611–617. [Google Scholar] [CrossRef] [PubMed]
- García-Aparicio, M.P.; Ballesteros, I.; González, A.; Oliva, J.M.; Ballesteros, M.; Negro, M.J. Effect of inhibitors released during steam-explosion pretreatment of barley straw on enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2006, 129, 278–288. [Google Scholar] [CrossRef]
- Larsson, S.; Reimann, A.; Nivelbrant, N.; Jonsson, L.J. Comparison of different methods for the detoxification of lignocellulose hydrolysates of spruce. Appl Biochem. Biotechnol. 1999, 77, 91–103. [Google Scholar] [CrossRef]
- Wilson, J.J.; Deschatelets, L.; Nishikawa, N.K. Comparative fermentability of enzymatic and acid hydrolysates of steam pretreated aspen wood hemicellulose by Pichia stipitis CBS 5776. Appl. Microbiol. Biotechnol. 1989, 31, 592–596. [Google Scholar] [CrossRef]
- Fargues, C.; Lewandowski, R.; Lameloise, M.L. Evaluation of ion-exchange and adsorbent resins for the detoxfication of beet distillery effluents. Ind. Eng. Chem. Res. 2010, 49, 9248–9257. [Google Scholar] [CrossRef]
- Rodrigues, R.; Felipe, M.G.A.; Silva, J.; Vitolo, M.; Gómez, P.V. The influence of pH, temperature and hydrolyzate concentration of volatile and nonvolatile compounds from sugarcane bagasse hemicellulosic hydrolyzate treate with charcoal before or after vacuum evaporation. Braz. J. Chem. Eng. 2001, 18, 299–311. [Google Scholar] [CrossRef]
- Alriksson, B.; Cavka, A.; Jhonson, L.F. Improving the fermentability of enzymatic hydrolysates of lignocellulose through chemical in situ modification with reducing agents. Bioresour. Technol. 2011, 102, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B.; Szengyel, Z.; Zacchi, G.; Réczey, K. Simultaneous detoxification and enzyme production of hemicellulose hydrolysates obtained after steam pretreatment. Enzyme Microbiol. Technol. 1997, 20, 286–293. [Google Scholar] [CrossRef]
- Okuda, N.; Soneura, M.; Ninomiya, K.; Katakura, Y.; Shioya, S. Biological detoxification of waste house wood hydrolysate using Ureibacillus thermosphaericus for bioethanol production. J. Biosci. Bioeng. 2008, 106, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.H.; Thygesen, A.; Thomsen, A.B. Identification and characterization of fermentation inhibitors formed during hydrothermal treatment and following SSF of wheat straw. Appl. Microbiol. Biotechonol. 2009, 83, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Adeboye, P.T.; Bettiga, M.; Aldaeus, F.; Larsson, P.T.; Olsson, L. Catabolism of coniferyl aldehyde, ferulic acid and p-coumaric acid by Saccharomyces cerevisiae yields less toxic products. Microb. Cell Fact. 2015, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Pejó, E.; Ballesteros, M.; Oliva, J.M.; Olsson, L. Adaptation of the xylose fermenting yeast Saccharomyces cerevisiae F12 for improving ethanol production in different fed-batch SSF processes. J. Ind. Microbiol. Biotechnol. 2010, 37, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Nivelbrant, N.O.; Jönsson, L.J. Effect of overexpression of Saccharomyces cerevisiae Pad1p on the resistance to phenylacrylic acids and lignocellulose hydrolysates under aerobic and oxygenlimited conditions. Appl. Microbiol. Biotechnol. 2001, 57, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Petersson, A.; Almeida, J.R.; Modig, T.; Karhumaa, K.; Hahn-Hägerdal, B.; Gorwa-Grauslund, M.F.; Lidén, G. A 5-hydroxymethyl furfural reducing enzyme encoded by the Saccharomyces cerevisiae ADH6 gene conveys HMF tolerance. Yeast 2006, 23, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.T.; Speranza, M.; Ruiz-Dueñas, F.J.; Ferreira, P.; Camarero, S.; Guillén, F.; Martínez, M.J.; Gutiérrez, A.; del Río, J.C. Biodegradation of lignocellulosics: Microbial, chemical and enzymatic aspects of fungal attack to lignin. Int. Microbiol. 2005, 8, 195–204. [Google Scholar] [PubMed]
- Gold, M.H.; Youngs, H.L.; Gelpke, M.D. Manganese peroxidase. Met. Ions Biol. Syst. 2000, 37, 559–586. [Google Scholar] [PubMed]
- Martínez, A.T. Molecular biology and structure-function of lignin degrading heme peroxidases. Enzym. Microb. Tehcnol. 2000, 30, 425–444. [Google Scholar] [CrossRef]
- Martínez, M.J.; Ruiz-Dueñas, F.J.; Guillén, F.; Martínez, A.T. Purification and catalytic properties of two manganese-peroxidase isoenzymes from Pleurotus eryngii. Eur. J. Biochem. 1996, 237, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Dueñas, F.J.; Martínez, M.J.; Martínez, A.T. Molecular characterization of a novel peroxidase isolated from the ligninolytic fungus Pleurotus eryingii. Mol. Microbiol. 1999, 31, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Salvachúa, D.; Prieto, A.; Martínez, A.T.; Martínez, M.J. Characterization of a novel DyP-type peroxidase from Irpex lacteus and its application in the enzymatic hydrolysis of wheat straw. Appl. Environ. Microbiol. 2013, 79, 4316–4324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Li, D.; Wang, L.J.; Wang, T.-P.; Zhang, L.; Dong Chen, X.; Mao, Z.-H. Effect of steam explosion on biodegradation of lignin in wheat straw. Bioresour. Technol. 2008, 99, 8512–8515. [Google Scholar] [CrossRef] [PubMed]
- Salvachúa, D.; Prieto, A.; López-Abelairas, M.; Lu-Chau, T.; Martínez, A.T.; Martínez, M.J. Fungal pretreatment: An alternative in second-generation ethanol form wheat straw. Bioresour. Technol. 2011, 102, 7500–7506. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, J.; He, J.; Liu, Z.; Yu, Z. Combinations of mild physical or chemical pretreatment with biological pretreatment for enzymatic hydrolysis of rice hull. Bioresour. Technol. 2009, 100, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sampedro, R.; Fillat, U.; Ibarra, D.; Eugenio, M.E. Use of new endophytic fungi as pretreatment to enhance enzymatic saccharification of Eucalyptus globulus. Bioresour. Technol. 2015, 196, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Suman, A.; Tiwari, P.; Arya, N.; Gaur, A.; Shrivastava, A.K. Biological pretreatment of sugarcane trash for its conversion to fermentable sugars. World J. Microbiol. Biotechnol. 2008, 24, 667–673. [Google Scholar] [CrossRef]
- Yoshida, H. Chemistry of lacquer (Urishi) part I. J. Chem. Soc. 1883, 43, 472–486. [Google Scholar] [CrossRef]
- Mayer, A.M.; Staples, R.C. Laccase: New function for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Fillat, U.; Martín-Sampedro, R.; Macaya-Sanz, D.; Martín, J.A.; Ibarra, D.; Martínez, M.J.; Eugenio, M.E. Screening of eucalyptus wood endophytes for laccase activity. Process Biochem. 2016, 51, 589–598. [Google Scholar] [CrossRef]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef]
- Morozova, O.; Shumakovich, G.; Gorbacheva, M.; Sheelev, S.; Yaropolov, A. “Blue laccases”. Biochemisery 2007, 72, 1136–1150. [Google Scholar] [CrossRef]
- Barreca, A.M.; Fabbrini, M.; Galli, C.; Gentili, P.; Ljunggren, S. Laccase/mediated oxidation of a lignin model for improved delignification procedures. J. Mol. Catal. B Enzym. 2003, 26, 105–110. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef]
- Cañas, A.I.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Call, H.-P. Verfahren zur Veränderung, Abbau oder Bleichen von Lignin, Ligninhaltigen Materialien oder Ähnlichen Stoffen. WO 1994029510 A1, 22 December 1994. [Google Scholar]
- Paice, M.G.; Bourbonnais, R.; Reid, I.; Archibald, F.S. Kraft pulp bleaching by redox enzymes. In Proceedings of the 9th International Symposium Wood Pulping Chemistry, Montreal, QC, Canada, 9–12 June 1997; pp. PL11–PL14. [Google Scholar]
- Camarero, S.; García, O.; Vidal, T.; Colom, J.; del Río, J.C.; Gutiérrez, A.; Gras, J.M.; Monje, R.; Martínez, M.J.; Martínez, A.T. Efficient bleaching of non-wood high-quality paper pulp using laccase–mediator system. Enzym. Microb. Technol. 2004, 35, 113–120. [Google Scholar] [CrossRef]
- Ibarra, D.; Camarero, S.; Romero, J.; Martínez, M.J.; Martínez, A.T. Integrating laccase–mediator treatment into an industrial-type sequence for totally chlorine free bleaching eucalypt kraft pulp. J. Chem. Technol. Biotechnol. 2006, 81, 1159–1165. [Google Scholar] [CrossRef]
- Kunamneni, A.; Plou, F.J.; Ballesteros, A.; Alcalde, M. Laccases and their applications: A patent review. Recent Pat. Biotechnol. 2008, 2, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Camarero, S.; Ibarra, D.; Martínez, M.J.; Martínez, A.T. Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl. Environ. Microbiol. 2005, 71, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Camarero, S.; Ibarra, D.; Martínez, A.T.; Romero, J.; Gutiérrez, A.; del Río, J.C. Paper pulp delignification using laccase and natural mediators. Enzym. Microb. Technol. 2007, 40, 1264–1271. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Rencoret, J.; Ibarra, D.; Molina, S.; Camarero, S.; Romero, J.; del Río, J.C.; Martínez, A.T. Removal of lipophilic extractives from paper pulp by laccase and lignin-derived phenols as natural mediators. Environ. Sci. Technol. 2007, 41, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.; Prieto, A.; Martínez-Alcalá, A.; Martínez, A.T.; Martínez, M.J. Laccase detoxification of steam-exploded wheat straw for second generation bioethanol. Bioresour. Technol. 2009, 100, 6378–6384. [Google Scholar] [CrossRef] [PubMed]
- Kolb, M.; Sieber, V.; Amann, M.; Faulstich, M.; Schieder, D. Removal of monomer delignification products by laccase from Trametes versicolor. Bioresour. Technol. 2012, 104, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Camarero, S.; Caña, A.I.; Nousiainen, P.; Record, E.; Lomascolo, A.; Martínez, M.J.; Martínez, A.T. p-Hydroxycinnamics acids as natural mediators of laccase detoxification of recalcitrant compounds. Environ. Sci. Technol. 2008, 42, 6703–6709. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, D.; Dhiman, S.S.; Kim, H.; Jeya, M.; Kim, I.-W.; Lee, J.-K. Characterization of a novel laccase from the isolated Coltricia perennis and its application to detoxification of biomass. Process Biochem. 2010, 47, 671–678. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Fernández, J.L.; Ballesteros, M. Different laccase detoxification strategies for ethanol production from lignocellulosic biomass by the thermotolerant yeas Kluyveromyces marxianus CECT 10875. Bioresour. Technol. 2012, 106, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Palmqvist, E.; Nivelbrant, N.-O.; Hahn-Hägerdal, B. Detoxification of wood hydrolysates with laccase and peroxidase from the white-rot fungus Trametes versicolor. Appl. Microbiol. Biotechnol. 1998, 49, 691–697. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Mialon, A.; Ballesteros, M. A bacterial laccase for enhancing saccharification and ethanol fermentation of steam-pretreated biomass. Fermentation 2016, 2, 11. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ibarra, D.; Ballesteros, I.; González, A.; Ballesteros, M. Comparing cell viability and ethanol fermentation of the thermotolerant yeast Kluyveromyces marxianus and Saccharomyces cerevisiae on steam-exploded biomass treated with laccase. Bioresour. Technol. 2013, 135, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. Exploring laccase and mediators behavior during saccharification and fermentation of steam-exploded wheat straw for bioethanol production. J. Chem. Technol. Biotechnol. 2016, 91, 1816–1825. [Google Scholar] [CrossRef]
- Martín, C.; Galbe, M.; Wahlbom, C.F.; Hahn-Hägerdal, B.; Jönsson, L. Ethanol production from enzymatic hydrolysates of sugarcane bagasse using recombinant xylose-utilising Saccharomyces cerevisiae. Enzym. Microb. Technol. 2002, 31, 274–282. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, X.; Chen, L.; Shen, Y.; Zhang, X.; Fang, W.; Wang, X.; Bao, X.; Xiao, Y. Identification of a laccase Glac15 from Ganoderma lucidum 77002 and its application in bioethanol production. Biotechnol. Biofuels 2015, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, J.; Zhou, P.; Wu, K.; Liu, H.; Xiong, C.; Gong, Y.; Xiao, W.; Liu, Z. One-pot simultaneous saccharification and fermentation: A preliminary study of a novel configuration for cellulosic ethanol production. Bioresour. Technol. 2014, 161, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Kapoor, R.K.; Singh, A.; Kuhad, R.C. Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour. Technol. 2007, 98, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Ballesteros, I.; Fernández, J.L.; Ballesteros, M. Ethanol from laccase-detoxified lignocellulose by the thermotolerant yeast Kluyveromyces marxianus—Effects of steam pretreatment conditions, process configurations and substrate loadings. Biochem. Eng. J. 2013, 79, 94–103. [Google Scholar] [CrossRef]
- Moreno, A.D.; Tomás-Pejó, E.; Ibarra, D.; Ballesteros, M.; Olsson, L. In situ laccase treatement enhances the fermentability of steam-exploded wheat straw in SSCF processes at high dry matter consistencies. Bioresour. Technol. 2013, 143, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.; Tomás-Pejó, E.; Ibarra, D.; Ballesteros, M.; Olsson, L. Fed-batch SSCF using steam-exploded wheat straw at high dry matter consistencies and a xylose-fermenting Saccharomyces ceresiae strain: Effect of laccase supplementation. Biotechnol. Biofuels 2013, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Moreno, A.D.; Ibarra, D.; Sáez, F.; Ballesteros, M. Improving the fermentation performance of Saccharomyces cerevisiae by laccase during ethanol production from steam-exploded wheat straw at high-substrate loadings. Biotechnol. Prog. 2013, 29, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Cassland, P.; Jönsson, L. Development of a Saccharomyces cerevisiae strain with enhanced resistance to phenolic fermentation inhibitors in lignocellulose hydrolysates by heterologous expression of laccase. Appl. Environ. Microbiol. 2001, 67, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.; Amann, M.; Hirth, T.; Rupp, S.; Zibek, S. Development and optimization of single and combined detoxification processes to improve the fermentability of lignocellulose hydrolyzates. Bioresour. Technol. 2013, 133, 455–461. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, M.; Martín-Sampedro, R.; Fillat, U.; Hernández, M.; Arias, M.E.; Eugenio, M.E.; Ibarra, D. Comparison of bacterial and fungal laccases for enhancing saccharification and ethanol fermentation of steam-pretreated biomass. In Proceedings of the IX Ibero-American Congress on Pulp and Paper Research—CIADICYP, Helsinki, Finland, 5–9 September 2016; p. 29. [Google Scholar]
- Enguita, F.J.; Matias, P.M.; Martins, L.O.; Placido, D.; Henriques, A.O.; Carrondo, M.A. Spore-coat laccase CotA from Bacillus subtilis: Crystallization and preliminary X-ray characterization by the MAD method. Acta Crystallogr. D Biol. Cristallogr. 2002, 58, 1490–1493. [Google Scholar] [CrossRef]
- Moya, R.; Saastamoinen, P.; Hernández, M.; Suurnäkki, A.; Arias, E.; Mattinen, M.-L. Reactivity of bacterial and fungal laccases with lignin under alkaline conditions. Bioresour. Technol. 2011, 102, 10006–10012. [Google Scholar] [CrossRef] [PubMed]
- Alfani, A.; Gallifuoco, F.F.; Saporosi, A.; Spera, A.; Cantarella, M. Comparison of SHF and SSF process for bioconversion of steam-exploded wheat straw. J. Ind. Microbiol. Biotechnol. 2000, 25, 184–192. [Google Scholar] [CrossRef]
- Abdel-Banat, B.M.A.; Hoshida, H.; Ano, A.; Hoshida, H.; Nonklang, S.; Akada, R. High-temperature fermentation: How can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl. Microbiol. Biotechnol. 2010, 85, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mudliar, S.; Sen, R.; Giri, B.; Staputti, D.; Chakrrabarti, T.; Pandey, R.A. Commercializing lignocellulosic bioethanol: Technology bottlenecks and possible remedies. Biofuels Bioprod. Biorefin. 2009, 4, 77–93. [Google Scholar] [CrossRef]
- Rosgaard, L.; Andric, P.; Dam-Johansen, K.; Pedersen, S.; Meyer, A.S. Effects of substrate loading on enzymatic hydrolysis and viscosity of pretreated barley straw to ethanol. Appl. Biochem. Biotechnol. 2007, 143, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Tabka, M.G.; Herpoel-Gimbert, I.; Monod, F.; Asther, M.; Sigoillot, J.C. Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulose, xylanase and feruloyl esterase treatment. Enzym. Microb. Technol. 2006, 39, 897–902. [Google Scholar] [CrossRef]
- Oliva-Taravilla, A.; Tomás-Pejó, E.; Demuez, M.; González-Fernández, C.; Ballesteros, M. Inhibition of cellulose enzymatic hydrolysis by laccase-derived compounds from phenols. Biotechnol. Prog. 2015, 31, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Taravilla, A.; Tomás-Pejó, E.; Demuez, M.; González-Fernández, C.; Ballesteros, M. Phenols and lignin: Key players in reducing enzymatic hydrolysis yields of steam-pretreated biomass in presence of laccase. J. Biotechnol. 2016, 218, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Taravilla, A.; Moreno, A.D.; Demuez, M.; Ibarra, D.; Tomás-Pejó, E.; González-Fernández, C.; Ballesteros, M. Unraveling the effects of laccase treatment on enzymatic hydrolysis of steam-exploded wheat straw. Bioresour. Technol. 2015, 175, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kuila, A.; Mukhopadhyay, M.; Tuli, D.K.; Banerjee, R. Accessibility of enzymatically delignified Bambusa bambos for efficient hydrolysis at minimum cellulase loading: An optimization study. Enzym. Res. 2011. [Google Scholar] [CrossRef] [PubMed]
- Kuila, A.; Mukhopadhyay, M.; Tuli, D.K.; Banerjee, R. Production of ethanol from lignocellulosics: An enzymatic venture. EXCLI J. 2011, 10, 85–96. [Google Scholar] [PubMed]
- Mukhopadhyay, M.; Kuila, A.; Tuli, D.K.; Banerjee, R. Enzymatic depolymerization of Ricinus communis, a potential lignocellulosic for improved saccharification. Biomass Bioenerg. 2011, 35, 3584–3591. [Google Scholar] [CrossRef]
- Rajak, R.C.; Banerjee, R. Enzyme mediated biomass pretreatment and hydrolysis: A biotechnological venture towards bioethanol production. RSC Adv. 2016, 6, 61301–61311. [Google Scholar] [CrossRef]
- Rencoret, J.; Pereira, A.; del Río, J.C.; Martínez, A.T.; Gutiérrez, A. Laccase-mediator pretreatment of wheat straw degrades lignin and improves saccharification. Bioenerg. Res. 2016, 9, 917–930. [Google Scholar] [CrossRef]
- Rico, A.; Rencoret, J.; del Río, J.C.; Martínez, A.T.; Gutiérrez, A. In-depth 2D NMR study of lignin modification during pretreatment of eucalyptus wood with laccase and mediators. Bioenerg. Res. 2015, 8, 211–230. [Google Scholar] [CrossRef]
- Singh, R.; Hu, J.; Regner, M.R.; Round, J.W.; Ralph, R.; Saddler, J.N.; Eltis, L.D. Enhanced delignification of steam-pretreated poplar by a bacterial laccase. Sci. Rep. 2017, 7, 42121. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, F.; Li, X.; Yan, Z.; Yuan, Y.; Liu, X.F. Enhanced saccharification of corn straw pretreated by alkali combining crude ligninolytic enzymes. J. Chem. Technol. Biotechnol. 2012, 87, 1687–1693. [Google Scholar] [CrossRef]
- Yang, P.; Jiang, S.; Zheng, Z.; Luo, S.; Pan, L. Effect of alkali and laccase pretreatment of Brassica campestris straw: Architecture, crystallisation, and saccharification. Polym. Renew. Resour. 2011, 2, 21–34. [Google Scholar]
- Qiu, W.; Chen, H. Enhanced the enzymatic hydrolysis efficiency of wheat straw after combined steam explosion and laccase pretreatment. Bioresour. Technol. 2012, 118, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Palonen, H.; Viikari, L. Role of oxidative enzymatic treatments on enzymatic hydrolysis of softwood. Biotechnol. Bioeng. 2004, 86, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, U.; Kellock, M.; Galkin, S.; Viikari, L. The laccase catalyzed modification of lignin for enzymatic hydrolysis. Enzym. Microb. Technol. 2011, 49, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, U.; Kellock, M.; Várnai, A.; Andberg, M.; Viikari, L. Mechanisms of laccase-mediator treatments improving the enzymatic hydrolysis of pre-treated spruce. Biotechnol. Biofuels 2014, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, A.K.; Mikkelsen, J.D.; Højrup, P.; Meyer, A.S. Identification of a laccase from Ganoderma lucidum CBS 229.93 having potential for enhancing cellulase catalyzed lignocellulose degradation. Enzym. Microb. Technol. 2013, 53, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qu, Y.; Xiao, P.; Wang, X.; Wang, T.; He, F. Improved biomass saccharification by Trichoderma reesei through heterologous expression of lacA gene Trametes sp. AH28-2. J. Biosci. Bioeng. 2012, 113, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Davidi, L.; Moraïs, S.; Artzi, L.; Knop, D.; Hadar, Y.; Arfi, Y.; Bayer, E.A. Toward combined delignification and saccharification of wheat straw by a laccase-containing designer cellulosome. Proc. Natl. Acad. Sci. USA 2016, 113, 10854–10859. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Hsieh, Z.-S.; Cheepudom, J.; Yang, C.-H.; Meng, M. A 24.7-kDa copper-containing oxidase, secreted by Thermobifida fusca, significantly increasing the xylanase/cellulase-catalyzed hydrolysis of sugarcane bagasse. Appl. Microbiol. Biotechnol. 2013, 97, 8977–8986. [Google Scholar] [CrossRef] [PubMed]
- Barneto, G.A.; Aracri, E.; Andreu, G.; Vidal, T. Investigating the structure-effect relationships of various natural phenols used as laccase mediators in the biobleaching of kenaf and sisal pulps. Bioresour. Technol. 2012, 112, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Zanirun, Z.; Bahrin, E.K.; Lai-Yee, P.; Hassan, M.; Abd-Aziz, S. Enhancement of fermentable sugars production from oil palm empty fruit bunch by ligninolytic enzymes mediator system. Int. Biodeterior. Biodegrad. 2015, 105, 13–20. [Google Scholar] [CrossRef]
- Lu, C.; Wang, H.; Luo, Y.; Guo, L. An efficient system for predelignification of gramineous biofuel feedstock in vitro: Application of a laccase from Pycnoporus sanguineus H275. Process Biochem. 2010, 45, 1141–1147. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Abualreesh, M.; Ahmed, K.; Razak, A.A. Enzymatic delignification of biomass for enhanced fermentable sugars production. Energy Technol. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Ono, T. Ionic liquid assisted enzymatic delignification of wood biomass: A new green and efficient approach for isolating of cellulose fibers. Biochem. Eng. J. 2012, 60, 156–160. [Google Scholar] [CrossRef]
- Financie, R.; Moniruzzaman, M.; Uemura, Y. Enhanced enzymatic delignification of oil palm biomass with ionic liquid pretreatment. Biochem. Eng. J. 2016, 110, 1–7. [Google Scholar] [CrossRef]
- Plácido, J.; Imam, T.; Capareda, S. Evaluation of ligninolytic enzymes, ultrasonication and liquid hot water as pretreatments for bioethanol production from cotton gin trash. Bioresour. Technol. 2013, 139, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Plácido, J.; Capareda, S. Analysis of ultrasonication pretreatment in bioethanol production from cotton gin trash using FTIR spectroscopy and principal component analysis. Bioresour. Bioprocess. 2014, 1, 1–9. [Google Scholar] [CrossRef]
- Nagula, K.N.; Pandit, A.B. Process intensification of delignification and enzymatic hydrolysis of delignified cellulosic biomass using various process intensification techniques including cavitation. Bioresour. Technol. 2016, 213, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; Rencoret, J.; Cadena, E.M.; Rico, A.; Barth, D.; del Río, J.C.; Martínez, A.T. Demonstration of laccase-based removal of lignin from wood and non-wood plant feedstocks. Bioresour. Technol. 2012, 119, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Rico, A.; Rencoret, J.; del Río, J.C.; Martínez, A.; Gutiérrez, A. Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock. Biotechnol. Bifuels 2014, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Al-Zuhair, S.; Ahmed, K.; Abdulrazak, A.; El-Nass, M.H. Synergistic effect of pretreatment and hydrolysis enzymes on the production of fermentable sugars from date palm lignocellulosic waste. J. Ind. Eng. Chem. 2013, 19, 413–415. [Google Scholar] [CrossRef]
- Chen, Q.; Marshall, N.M.; Geib, S.M.; Tien, M.; Richard, T.L. Effects of laccase on lignin depolymerization and enzymatic hydrolysis of ensiled corn stover. Bioresour. Technol. 2012, 117, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Heap, L.; Green, A.; Brown, D.; Dongen, B.V.; Turner, N. Role of laccase as an enzymatic pretreatment method to improve lignocellulosic saccharification. Catal. Sci. Technol. 2014, 4, 2251–2259. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Capanema, E.A.; Hoeger, I.; Villar, J.C.; Rojas, O.J. Lignin changes after steam explosion and laccase-mediator treatment of eucalyptus wood chips. J. Agric. Food. Chem. 2011, 59, 8761–8769. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sampedro, R.; Eugenio, M.E.; García, J.C.; López, F.; Villar, J.C.; Diaz, M.J. Steam explosion and enzymatic pre-treatments as an approach to improve the enzymatic hydrolysis of Eucalyptus globulus. Biomass Bioeng. 2012, 42, 97–106. [Google Scholar] [CrossRef]
- Milstein, O.; Haars, A.; Majchercyk, A.; Tautz, D.; Zanker, H.; Hüttermann, A. Removal of chlorophenols and chlorolignins from bleaching effluents by combining chemical and biological treatment. Water Sci. Technol. 1988, 20, 161–170. [Google Scholar]
- Widsten, P.; Kandelbauer, A. Adhesion improvement of lignocellulosic products by enzymatic pre-treatment. Biotechnol. Adv. 2008, 26, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.P.; Ragauskas, A.J. Evaluating laccase-facilitated coupling of phenolic acids to high-yield kraft pulps. Enzym. Microb. Technol. 2002, 30, 855–861. [Google Scholar] [CrossRef]
- Elegir, G.; Kindl, A.; Sadocco, P.; Orlandi, M. Development of antimicrobial cellulose packaging through laccase-mediated grafting of phenolic compounds. Enzym. Microb. Technol. 2008, 43, 84–92. [Google Scholar] [CrossRef]
- Fillat, A.; Gallardo, O.; Vidal, T.; Pastor, F.I.J.; Díaz, P.; Roncero, M.B. Enzymatic grafting of natural phenols to flax fibres: Development of antimicrobial properties. Carbohydr. Polym. 2012, 87, 146–152. [Google Scholar] [CrossRef]
- Roth, S.; Spiess, A.C. Laccases for biorefinery applications: A critical review on challenges and perspectives. Bioprocess Biosyst. Eng. 2015, 38, 2285–2313. [Google Scholar] [CrossRef] [PubMed]



| Pretreated Material | Laccase Treatment | Effects Observed | Benefits Produced | Reference |
|---|---|---|---|---|
| Steam-exploded rice straw | Coltricia perennis | Removal of phenolic compounds by 76% | Increased saccharification yield by 48% | [79] |
| Steam-exploded wheat straw | Pycnoporus cinnabarinus or Trametes villosa | Removal of phenols identified (vanillin, syringaldehyde, ferulic acid and p-coumaric acid) by 93–95% with both laccases | Improved the fermentation performance of Kluyveromyces marxianus CECT 10875, shortening its lag phase and enhancing the ethanol yields | [80] |
| SO2 steam-pretreated willow | Trametes versicolor | Removal of phenolic compounds (93–95%), revealing an oxidative polymerization mechanism by SEC analysis | Higher yeast growth, glucose consumption rate, ethanol productivity and ethanol yield using Saccharomyces cerevisiae | [81] |
| Dilute acid steam-pretreated spruce | T. versicolor | Removal of phenolic compounds by 93–95% | Ethanol yield produced by S. cerevisiae comparable with that obtained after detoxification with anion exchange chromatography at pH 10 | [37] |
| Steam-exploded wheat straw | Commercial bacterial laccase MetZyme® | Phenol reduction of 18% (laccase alone) and 21% (simultaneous laccase and presaccharification) | Improved the fermentation performance of K. marxianus CECT 10875 during SSF and PSSF processes, shortening the adaptation phases and the overall fermentation times | [82] |
| Water and acid-impregnated steam-exploded wheat straw | T. versicolor or Coriolopsis rigida | Removal of phenolic compounds by 93–95% with both laccases | Reduction of the toxic effects on S. cerevisiae, resulting in higher yeast growth and improved ethanol production | [76] |
| Steam-exploded wheat straw | P. cinnabarinus | Phenol reduction around 67% (laccase alone) and 73% (simultaneous laccase and presaccharification) | Laccase detoxification allowed to obtain ethanol concentrations and yields with K. marxianus CECT 10875 comparable to those obtained with S. cerevisiae | [83] |
| Steam-exploded wheat straw | P. cinnabarinus | Removal of phenolic compounds by 95% | Improvement of cell growth and ethanol production of S. cerevisiae during SSF process | [84] |
| Steam-exploded sugarcane bagasse | T. versicolor | Approximately 80% of the phenolic compounds removal | Improvements in ethanol yield and ethanol volumetric using a xylose-utilizing S. cerevisiae | [85] |
| Steam-exploded sugarcane bagasse | Ganoderma lucidum 77002 | 84% of the phenolic compounds in prehydrolysate | Ethanol yield was improved when S. cerevisiae was used on detoxified prehydrolysate | [86] |
| Alkali-extracted sugarcane bagasse | Aspergillus oryzae | Not observed | Laccase improved the fermentation efficiency by 6.8% for one-pot SSF and 5.7% for SSF | [87] |
| Acid hydrolyzed from sugarcane bagasse | Cyathus stercoreus | Reduction of 77.5% of total phenols | Improvements in the performance of Candida shehatae NCIM 3501 | [88] |
| Steam-exploded wheat straw | P. cinnabarinus | Phenol reduction around 44% (laccase alone) and 95% (simultaneous laccase and presaccharification) at 12% (w/v) of substrate loading | Laccase detoxification triggered the fermentation by K. marxianus of steam-exploded material at 12% (w/v), resulting in an ethanol concentration of 16.7 g/L during SSF process | [89] |
| Steam-exploded wheat straw | P. cinnabarinus | Reduction of total phenolic compounds by 50–80% | Laccase detoxification allowed the fermentation of pretreated material at 20% (w/v) of substrate loading using the evolved xylose-consuming yeast S. cerevisiae F12, producing more than 22 g/L during SSCF process | [90] |
| Steam-exploded wheat straw | P. cinnabarinus | Approximately 73–81% of the phenolic compounds removal | Laccase detoxification improved cell viability of the evolved xylose-recombinant S. cerevisiae KE6-12, and increased the ethanol production up to 32 g/L when fed-batch SSCF process was used at 16% (w/v) of substrate loading | [91] |
| Steam-exploded wheat straw | P. cinnabarinus | Phenols removal by 53% during simultaneous laccase and presaccharification at 25% (w/v) of substrate loading | Ethanol production of 58.6 g/L at 48 h with detoxified material at 25% (w/v) of substrate loading during PSSF process with S. cerevisiae | [92] |
| Dilute-acid spruce hydrolysate | T. versicolor expressed in a recombinant S. cerevisiae strain | Reduction of low-molecular of phenolic compounds | Laccase-producing transformant was able to ferment at a faster rate than the control transformant | [93] |
| Organosolv pretreated wheat straw | T. versicolor immobilized on both active epoxide and amino carriers | Higher phenols removal (82%) efficiency with laccase immobilized on active amino carrier | Better performance of Pichia stipitis during fermentation and reusability of immobilized laccase | [94] |
| Steam-exploded wheat straw | T. villosa or a bacterial laccase from Streptomyces ipomoeae | Phenol content reduction of 29% and 90% with bacterial and fungal laccases, respectively | Improvement performance of S. cerevisiae during SSF and PSSF process | [95] |
| Pretreated Material | Laccase Treatment | Effects Observed | Benefits Produced | Reference |
|---|---|---|---|---|
| Milled material from Thorny bamboo and Spanish flag | Pleurotus sp. | Range of delignification between 84–89%, revealing the lignin removal by FTIR, XRD, and SEM analysis | Better accessibility of hydrolytic enzymes | [106,107] |
| Milled material from Ricinus communis | Pleurotus sp. | 86% of lignin loss, resulting in a degradation of the surface tissues (SEM analysis) | Reducing sugar yields increased 2.68-fold | [108] |
| Milled material from karn grass | Lentinus squarrosulus MR13 | Lignin removal of 81.6%. Porosity analysis evidenced the specific action of laccase on lignin | Increase of sugar production of 7.03 fold | [109] |
| Milled material from wheat straw | P. cinnabarinus laccase followed by alkaline peroxide extraction | 18% decrease in lignin after sequential treatment | 24–25% increase in glucose and xylose production | [110] |
| Milled wood from Eucalyptus globulus | Four cycles of Myceliophthora thermophila laccase -alkaline extraction | Up to 20% of lignin loss after four cycles treatment | Increase of glucose production by 9% | [111] |
| Steam-pretreated poplar | Bacterial laccase from Amycolatopsis sp. | Increment of acid insoluble lignin release by 6 fold, observing a reduction of molar mass lignin (approx. 50%) by SEC analysis | 8% increment of glucose production | [112] |
| Alkali-extracted corn straw | Trametes hirsuta | Increment of porositiy and surface area in laccase-treated samples | 2-fold increment in sugar production | [113] |
| Alkali-extracted straw from Brassica campestris | Ganoderma lucidum | Higher number and density of holes with greater width and depth after laccase treatment | Saccharification yield increased 1.7-fold | [114] |
| Steam-exploded wheat straw | Sclerotium sp. | Loosening of lignin-carbohydrate complex | 16.8% increase in cellulose hydrolysis | [115] |
| Acid steam-pretreated spruce | T. hirsuta | Reduction of lignin hydrophobicity and enrichment of carboxylic groups revealed by ESCA (electron spectroscopy for chemical analysis) | 13% increase in sugar yield | [116] |
| Acid steam-pretreated spruce | Cerrena unicolor and T. hirsuta laccases | Reduced binding of hydrolytic enzymes by lignin modification | Improvement of hydrolysis yield by 12% | [117,118] |
| Steam-exploded sugarcane bagasse | G. lucidum | Delignification | 75% increase in glucose production | [119] |
| Corncob residue | Trametes sp. AH28-2 heterologously expressed in Trichoderma reesei | Not investigated | Up to 71.6% increase in reducing sugar yields | [120] |
| Milled wheat straw | Bacterial laccase from Thermobifida fusca incorporated into a designer cellulosome including two cellulases and xylanase | Not investigated | Reducing sugar yields increased 2.0-fold | [121] |
| Milled sugarcane bagasse | Bacterial laccase from T. fusca | SEM analysis of laccase-treated sample shows smaller shatters | 2-fold increment in sugar production | [122] |
| Steam-exploded wheat straw | Alkaline extraction followed by a commercial bacterial laccase MetZyme® | Slight delignification (2%) after alkaline extraction-laccase sequence | Increment of glucose and xylose production by 21% and 30%, respectively | [82] |
| Steam-exploded wheat straw | Alkaline extraction followed by Trametes villosa laccase or bacterial laccase from Streptomyces ipomoeae treatment | Slight delignification (4%) after alkaline extraction-laccase sequence. No delignification observed by T. villosa | Increment of glucose and xylose production by 16% and 6%, respectively. No positive effects observed by T. villosa | [95] |
| Pretreated Material | LMS Treatment | Effects Observed | Benefits Produced | Reference |
|---|---|---|---|---|
| Oil palm empty fruit bunch milled | Pycnoporus sanguineus laccase with HBT and ABTS as mediators | Klason lignin reduction of 8% and 8.7% for HBT and ABTS, respectively | Increment of sugar yield by 16–17% compared to laccase alone | [124] |
| Wheat straw and corn stover pretreated with liquid hot water | P. sanguineus H275 laccase with VIO as mediator | Up to 97% lignin loss | 19.98% increase in sugar production | [125] |
| Milled material from palm trees and seaweed | Trametes versicolor laccase with HBT as mediator | Lignin removal of 9% and 24% for palm trees and seaweed, respectively | Better enzymatic hydrolysis with a ionic liquid [C2 mim] [OAc] (1-ethyl-3-methylimidazolium acetate) treatment prior to laccase-HBT | [126] |
| Wood chips swollen with ionic liquid [C2 mim] [OAc] (1-ethyl-3-methylimidazolium acetate) | Trametes sp. Y120 laccase with HBT as mediator | 50% delignification, revealing structural lignin changes by SEM and FTIR analysis | Pretreated material with cellulose more accessible | [127] |
| Oil palm empty fruit bunch pre-treated with ionic liquid [EMIM] [DEP] (1-ethyl-3-methylimidazolium diethyl phosphate) | Trametes sp. Y120 laccase with HBT as mediator | 35% decrease in lignin | Cellulose rich-material | [128] |
| Cotton gin trash pretreated with a sequential combination of ultrasonication and liquid hot water | Cerrena unicolor laccase with 3,5-dimethoxy-4-hydroxybenzonitrile as mediator | Up to 15% lignin loss | Up to 23% and 31% increase in glucose and ethanol yields, respectively | [129] |
| Cotton gin trash pretreated with a sequential combination of alkaline ultrasonication and liquid hot water | C. unicolor laccase with 3,5-dimethoxy-4-hydroxybenzonitrile as mediator | 27% reduction in lignin, observing lignin aromatic change structure by FTIR | 41% and 64% increase in glucose and ethanol yields, respectively | [130] |
| Elephant grass pretreated with ultrasond | Trametes hirsuta laccase with ABTS as mediator | Delignification range of 69% | Better accessibility of cellulose | [131] |
| Milled materials from eucalypt wood and elephant grass | Trametes villosa laccase with HBT as mediator and a subsequent alkaline extraction | Up to 48% and 32% lignin removal for eucalypt and elephant grass, respectively | Increase in glucose yield (61% and 12% for eucalypt and elephant grass, respectively) and ethanol production (over 4 g/L in eucalypt and 2 g/L in elephant) | [132] |
| Eucalypt wood milled | Four cycles of Myceliophthora thermophila laccase with methyl syringate as mediator and a subsequent alkaline peroxide extraction | 50% delignification, observing by Py/GC-MS and 2D NMR analysis a significant reduction of both aromatic and aliphatic lignin with high presence of oxidized syringyl units | Increases (approximately 40%) in glucose and xylose yields after enzymatic hydrolysis | [133] |
| Eucalypt wood milled | Comparing four cycles of Pycnoporus cinnabarinus laccase with HBT as mediator (or M. thermophila laccase with methyl syringate as mediator) and a subsequent alkaline peroxide extraction | 50% decrease in lignin with both LMS after four cycles, Slight delignification observed after the first cycle with P. cinnabarinus laccase and HBT, but not after M. thermophila laccase and methyl syringate | Increased glucose yield (30%) with both LMS after four cycles Saccharification increment of 10% after the first cycle with P. cinnabarinus laccase and HBT, but not after M. thermophila laccase and methyl syringate | [111] |
| Acid steam-pretreated spruce | T. hirsuta laccase with acetosyringone as mediator | Reduction of unproductive hydrolases adsorption due to an increment of syringyl/guaiacyl ratio | Downstream cellulose hydrolysis was improved 36% | [118] |
| Acid steam-pretreated spruce | T. hirsuta laccase with ABTS, HBT, and TEMPO as mediators | Lignin modification resulting in a decrease of unproductive cellulases adsorption, except with HBT. TEMPO also oxidized cellulose | Increment of enzymatic hydrolysis by 54% and 49% with ABTS and TEMPO, respectively. No positive effects with HBT | [118] |
| Milled material from date palm waste | T. versicolor laccase with HBT as mediator | Reduced binding of hydrolytic enzymes by lignin modification | Improvement of sugar production 8 times | [134] |
| Ensiled corn stover | T. versicolor laccase with HBT as mediator | Lignin side chain oxidation | Downstream cellulose hydrolysis was improved 7% | [135] |
| Acid steam-exploded wheat straw | T. versicolor laccase with HBT as mediator followed by alkaline peroxide extraction | Lignin oxidation revealed by Py/GC-MS TMAH | Increment of glucose release by up to 2.3 g/L | [136] |
| Acid steam-pretreated spruce | T. hirsuta laccase with NHA as mediator | Lignin modification showing both modified hydrophobicity and surface charge | Enzymatic hydrolysis yield increased 1.61-fold compared to laccase alone | [116] |
| Steam-exploded eucalypt wood | M. thermophila laccase and HBT as mediator | Lignin oxidation let to an increment of both secondary OH groups and degree condensation | Slightly increase of sugar production | [137,138] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fillat, Ú.; Ibarra, D.; Eugenio, M.E.; Moreno, A.D.; Tomás-Pejó, E.; Martín-Sampedro, R. Laccases as a Potential Tool for the Efficient Conversion of Lignocellulosic Biomass: A Review. Fermentation 2017, 3, 17. https://doi.org/10.3390/fermentation3020017
Fillat Ú, Ibarra D, Eugenio ME, Moreno AD, Tomás-Pejó E, Martín-Sampedro R. Laccases as a Potential Tool for the Efficient Conversion of Lignocellulosic Biomass: A Review. Fermentation. 2017; 3(2):17. https://doi.org/10.3390/fermentation3020017
Chicago/Turabian StyleFillat, Úrsula, David Ibarra, María E. Eugenio, Antonio D. Moreno, Elia Tomás-Pejó, and Raquel Martín-Sampedro. 2017. "Laccases as a Potential Tool for the Efficient Conversion of Lignocellulosic Biomass: A Review" Fermentation 3, no. 2: 17. https://doi.org/10.3390/fermentation3020017
APA StyleFillat, Ú., Ibarra, D., Eugenio, M. E., Moreno, A. D., Tomás-Pejó, E., & Martín-Sampedro, R. (2017). Laccases as a Potential Tool for the Efficient Conversion of Lignocellulosic Biomass: A Review. Fermentation, 3(2), 17. https://doi.org/10.3390/fermentation3020017