Influence of Media Heat Sterilization Process on Growth Performance of Representative Strains of the Genus Lactobacillus
Abstract
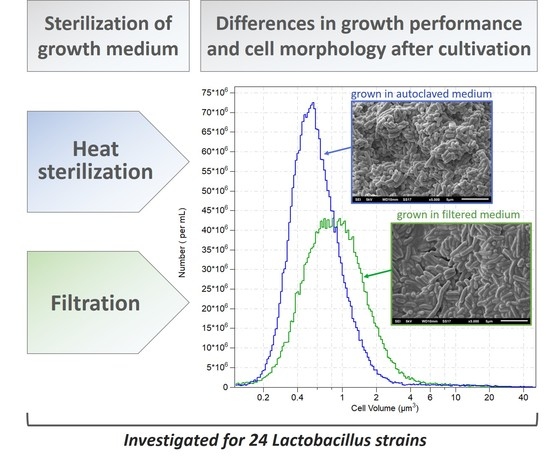
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Culture Media
2.3. Culture Conditions
2.4. Determination of the Total Cell Concentration and Cell Volume Distribution
2.5. Scanning Electron Microscope Images
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arena, M.P.; Caggianiello, G.; Russo, P.; Albenzio, M.; Massa, S.; Fiocco, D.; Capozzi, V.; Spano, G. Functional Starters for Functional Yogurt. Foods 2015, 4, 15–33. [Google Scholar] [CrossRef] [Green Version]
- Pasini, F.; Soglia, F.; Petracci, M.; Caboni, M.F.; Marziali, S.; Montanari, C.; Gardini, F.; Grazia, L.; Tabanelli, G. Effect of Fermentation with Different Lactic Acid Bacteria Starter Cultures on Biogenic Amine Content and Ripening Patterns in Dry Fermented Sausages. Nutrients 2018, 10, 1497. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Fares, C.; Longo, A.; Spano, G.; Capozzi, V. Lactobacillus plantarum with Broad Antifungal Activity as a Protective Starter Culture for Bread Production. Foods 2017, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; van Sinderen, D.; O’Toole, P.W. Lactobacillus phylogenomics—Towards a reclassification of the genus. Int. J. Syst. Evol. Microbiol. 2008, 58, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.; Plummer, S.; Marchesi, J.; Mahenthiralingam, E. The life history of Lactobacillus acidophilus as a probiotic: A tale of revisionary taxonomy, misidentification and commercial success. FEMS Microbiol. Lett. 2013, 349, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, E.; Torriani, S.; Felis, G.E. The genus Lactobacillus: A taxonomic update. Probiot. Antimicrob. Proteins 2012, 4, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, E.; O’Toole, P.W. When regulation challenges innovation: The case of the genus Lactobacillus. Trends Food Sci. Technol. 2017, 66, 187–194. [Google Scholar] [CrossRef]
- Cappello, M.S.; Zapparoli, G.; Logrieco, A.; Bartowsky, E.J. Linking wine lactic acid bacteria diversity with wine aroma and flavour. Int. J. Food Microbiol. 2017, 243, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Nionelli, L.; Montemurro, M.; Pontonio, E.; Verni, M.; Gobbetti, M.; Rizzello, C.G. Pro-technological and functional characterization of lactic acid bacteria to be used as starters for hemp (Cannabis sativa L.) sourdough fermentation and wheat bread fortification. Int. J. Food Microbiol. 2018, 279, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Romanens, E.; Freimüller Leischtfeld, S.; Volland, A.; Stevens, M.J.A.; Krähenmann, U.; Isele, D.; Fischer, B.; Meile, L.; Miescher Schwenninger, S. Screening of lactic acid bacteria and yeast strains to select adapted anti-fungal co-cultures for cocoa bean fermentation. Int. J. Food Microbiol. 2019, 290, 262–272. [Google Scholar] [CrossRef]
- Wright, C.T.; Klaenhammer, T.R. Calcium-induced alteration of cellular morphology affecting the resistance of Lactobacillus acidophilus to freezing. Appl. Environ. Microbiol. 1981, 41, 807–815. [Google Scholar] [PubMed]
- Wright, C.T.; Klaenhammer, T.R. Influence of calcium and manganese on dechaining of Lactobacillus bulgaricus. Appl. Environ. Microbiol. 1983, 46, 785–792. [Google Scholar] [PubMed]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, I.; Kitahara, K. Effect of several vitamins on the cell division and the growth of Lactobacillus delbrueckii. J. Vitaminol. 1962, 8, 115–120. [Google Scholar] [CrossRef]
- Deibel, R.H.; Downing, M.; Niven, C.F., Jr.; Schweigert, B.S. Filament formation by Lactobacillus leichmannii when desoxyribosides replace vitamin B12 in the growth medium. J. Bacteriol. 1956, 71, 255–256. [Google Scholar] [PubMed]
- Ives, D.H.; Ikeda, S. Life on the salvage path: Deoxynucleoside kinases of Lactobacillus acidophilus R-26. Prog. Nucleic Acid Res. Mol. Biol. 1997, 59, 205–255. [Google Scholar] [CrossRef]
- Reich, J.; Soska, J. Thymineless death in Lactobacillus acidophilus R-26. II. Factors determining the rate of the reproductive inactivation. Folia Microbiol. (Praha) 1973, 18, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Jeener, H.; Jeener, R. Cytological study of Thermobacterium acidophilus R 26 cultured in absence of deoxyribonucleosides or uracil. Exp. Cell Res. 1952, 3, 675–680. [Google Scholar] [CrossRef]
- Jacques, N.A.; Hardy, L.; Knox, K.W.; Wicken, A.J. Effect of Tween 80 on the morphology and physiology of Lactobacillus salivarius strain IV CL-37 grown in a chemostat under glucose limitation. J. Gen. Microbiol. 1980, 119, 195–201. [Google Scholar] [CrossRef]
- Senz, M.; van Lengerich, B.; Bader, J.; Stahl, U. Control of cell morphology of probiotic Lactobacillus acidophilus for enhanced cell stability during industrial processing. Int. J. Food Microbiol. 2015, 192, 34–42. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M. A medium for the cultivation of lactobacilli. J. Appl. Microbiol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Felis, G.E.; Dellaglio, F. Taxonomy of Lactobacilli and Bifidobacteria. Curr. Issues Intest. Microbiol. 2007, 8, 44–61. [Google Scholar] [PubMed]
- Vos, P.; Garrity, G.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A.; Schleifer, K.-H.; Whitman, W.B. Bergey’s Manual of Systematic Bacteriology: Volume 3: The Firmicutes; Springer Science & Business Media: New York, NY, USA, 2011. [Google Scholar]
- Bylund, G. Dairy Processing Handbook; Tetra Pak Processing Systems AB: Lund, Sweden, 1995. [Google Scholar]
- Hellwig, M.; Henle, T. Baking, ageing, diabetes: A short history of the Maillard reaction. Angew. Chem. Int. Ed. 2014, 53, 10316–10329. [Google Scholar] [CrossRef] [PubMed]
- Rurián-Henares, J.A.; Morales, F.J. Antimicrobial activity of melanoidins against Escherichia coli is mediated by a membrane-damage mechanism. J. Agric. Food Chem. 2008, 56, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Lee, S.B. Inhibitory Effect of Maillard reaction products on growth of the aerobic marine hyperthermophilic archaeon Aeropyrum pernix. Appl. Environ. Microbiol. 2003, 69, 4325. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martínez, M.; Ávila, M.; Moreno, F.J.; Requena, T.; Villamiel, M. Effect of milk protein glycation and gastrointestinal digestion on the growth of bifidobacteria and lactic acid bacteria. Int. J. Food Microbiol. 2012, 153, 420–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parlindungan, E.; Dekiwadia, C.; Tran, K.T.M.; Jones, O.A.H.; May, B.K. Morphological and ultrastructural changes in Lactobacillus plantarum B21 as an indicator of nutrient stress. LWT 2018, 92, 556–563. [Google Scholar] [CrossRef]
- Hébert, E.M.; Raya, R.R.; de Giori, G.S. Nutritional requirements of Lactobacillus delbrueckii subsp. lactis in a chemically defined medium. Curr. Microbiol. 2004, 49, 341–345. [Google Scholar] [CrossRef]
- Møretrø, T.; Hagen, B.F.; Axelsson, L. A new, completely defined medium for meat lactobacilli. J. Appl. Microbiol. 2004, 85, 715–722. [Google Scholar] [CrossRef]
- Foster, E.M. The effect of heat on milk as a Culture medium for lactic acid bacteria. J. Dairy Sci. 1952, 35, 988–997. [Google Scholar] [CrossRef]
- Stulova, I.; Kabanova, N.; Kriščiunaite, T.; Laht, T.M.; Vilu, R. The effect of milk heat treatment on the growth characteristics of lactic acid bacteria. Agron. Res. 2011, 9, 473–478. [Google Scholar]





| Name | Applied Growth Temperature | Phylogenetic Group * | Main Habitat or Source of Isolation ** | Type of Fermentation ** |
|---|---|---|---|---|
| L. acidophilus La-0103 | 37 °C | delb | I, F | a |
| L. acidophilus La-0107 | 37 °C | delb | I, F | a |
| L. acidophilus NCFM | 37 °C | delb | I, F | a |
| L. brevis La-0410 | 30 °C | bre | F, D | c |
| L. buchneri La-1501 | 37 °C | buch | F, D | c |
| L. casei subsp. alactosus La-0605 | 30 °C | cas | F | b |
| L. casei subsp. casei La-0618 | 30 °C, 37 °C | cas | F | b |
| L. confusus La-2201 (Weissella confusa) | 30 °C | - | F, I, S | c |
| L. curvatus La-1001 | 30 °C | sakei | I, F, D | b |
| L. delbrueckii subsp. bulgaricus La-0502 | 37 °C | delb | F | a |
| L. delbrueckii subsp. delbrueckii La-0704 | 37 °C | delb | F | a |
| L. delbrueckii subsp. lactis La-07/102 | 37 °C | delb | F | a |
| L. farciminis La-3401 | 30 °C | al-far | F | a |
| L. johnsonii La-2801 | 37 °C | delb | I, F | a |
| L. kefiri La-2601 | 30 °C | buch | F | c |
| L. pentosus La-3301 | 30 °C | plan | F, S | b |
| L. plantarum La-1203 | 30 °C | plan | F, D | b |
| L. plantarum La-1210 | 37 °C | plan | F, D | b |
| L. rhamnosus La-0610 | 37 °C | cas | F | b |
| L. rhamnosus La-0611 | 37 °C | cas | F | b |
| L. rhamnosus La-0617 | 37 °C | cas | F | b |
| L. rhamnosus La-0619 | 37 °C | cas | F | b |
| L. ruminis La-2101 | 37 °C | sal | I | a |
| L. salivarius subsp. salivarius La-2002 | 37 °C | sal | I | a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senz, M.; Keil, C.; Schmacht, M.; Palinski, S.; Cämmerer, B.; Hageböck, M. Influence of Media Heat Sterilization Process on Growth Performance of Representative Strains of the Genus Lactobacillus. Fermentation 2019, 5, 20. https://doi.org/10.3390/fermentation5010020
Senz M, Keil C, Schmacht M, Palinski S, Cämmerer B, Hageböck M. Influence of Media Heat Sterilization Process on Growth Performance of Representative Strains of the Genus Lactobacillus. Fermentation. 2019; 5(1):20. https://doi.org/10.3390/fermentation5010020
Chicago/Turabian StyleSenz, Martin, Claudia Keil, Maximilian Schmacht, Sophie Palinski, Bettina Cämmerer, and Martin Hageböck. 2019. "Influence of Media Heat Sterilization Process on Growth Performance of Representative Strains of the Genus Lactobacillus" Fermentation 5, no. 1: 20. https://doi.org/10.3390/fermentation5010020
APA StyleSenz, M., Keil, C., Schmacht, M., Palinski, S., Cämmerer, B., & Hageböck, M. (2019). Influence of Media Heat Sterilization Process on Growth Performance of Representative Strains of the Genus Lactobacillus. Fermentation, 5(1), 20. https://doi.org/10.3390/fermentation5010020