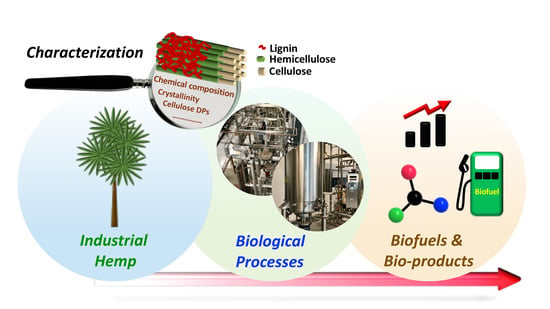
Recent Advancements in Biological Conversion of Industrial Hemp for Biofuel and Value-Added Products
Abstract
:1. Introduction
2. Characteristics of Industrial Hemp
2.1. Chemical Composition of Industrial Hemp
2.2. Morphological Properties of Industrial Hemp
2.3. Other Characteristics of Industrial Hemp: Crystallinity and Degree of Polymerization of Cellulose
3. Biological Conversion Approaches with Industrial Hemp
3.1. Pretreatment Strategies for Industrial Hemp
3.2. Enzymatic Hydrolysis of Industrial Hemp for Sugar Production
3.3. Fermentation
3.3.1. Bioethanol Production
3.3.2. Succinic Acid
3.3.3. Poly-3-Hydroxybutyrate P(3HB)
3.3.4. Anaerobic Digestion of Hemp for Biogas Production
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The Seed of Industrial Hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hempseed in food industry: Nutritional value, health benefits, and industrial applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 282–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zając, M.; Guzik, P.; Kulawik, P.; Tkaczewska, J.; Florkiewicz, A.; Migdał, W. The quality of pork loaves with the addition of hemp seeds, de-hulled hemp seeds, hemp protein and hemp flour. LWT 2019, 105, 190–199. [Google Scholar] [CrossRef]
- Devi, V.; Khanam, S. Comparative study of different extraction processes for hemp (Cannabis sativa) seed oil considering physical, chemical and industrial-scale economic aspects. J. Cleaner Prod. 2019, 207, 645–657. [Google Scholar] [CrossRef]
- RES. The Species Problem in Cannabis: Science and Semantics; JSTOR: New York, NY, USA, 1979; Volume 1. [Google Scholar]
- Cherney, J.H.; Small, E. Industrial hemp in North America: Production, politics and potential. Agronomy 2016, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Tehsin, Z.; Malik, S.T.; Asad, S.A.; Shahzad, M.; Bilal, M.; Shah, M.M.; Khan, S.A. Phytoremediation Potential of Hemp (Cannabis sativaL.): Identification and Characterization of Heavy Metals Responsive Genes. CLEAN-Soil Air Water 2016, 44, 195–201. [Google Scholar] [CrossRef]
- Stevulova, N.; Cigasova, J.; Estokova, A.; Terpakova, E.; Geffert, A.; Kacik, F.; Singovszka, E.; Holub, M. Properties characterization of chemically modified hemp hurds. Materials 2014, 7, 8131–8150. [Google Scholar] [CrossRef]
- Van der Werf, H.M.; van der Veen, J.H.; Bouma, A.; Ten Cate, M. Quality of hemp (Cannabis sativa L.) stems as a raw material for paper. Ind. Crops Prod. 1994, 2, 219–227. [Google Scholar] [CrossRef]
- Wang, H.; Postle, R.; Kessler, R.; Kessler, W. Removing pectin and lignin during chemical processing of hemp for textile applications. Text. Res. J. 2003, 73, 664–669. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Picandet, V.; Amziane, S.; Baley, C. Influence of compactness and hemp hurd characteristics on the mechanical properties of lime and hemp concrete. Eur. J. Environ. Civ. Eng. 2009, 13, 1039–1050. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Roozeboom, K.; Wang, D. Bioconversion of industrial hemp biomass for bioethanol production: A review. Fuel 2020, 281, 118725. [Google Scholar] [CrossRef]
- Das, L.; Li, W.; Dodge, L.A.; Stevens, J.C.; Williams, D.W.; Hu, H.; Li, C.; Ray, A.E.; Shi, J. Comparative Evaluation of Industrial Hemp Cultivars: Agronomical Practices, Feedstock Characterization, and Potential for Biofuels and Bioproducts. ACS Sustainable Chem. Eng. 2020, 8, 6200–6210. [Google Scholar] [CrossRef]
- Coit, M. The Fate of Industrial Hemp in the 2018 Farm Bill-Will Our Collective Ambivalence Finally be Resolved? J. Food L. Pol’y 2018, 14, 12. [Google Scholar]
- Johnson, R. Hemp as an Agricultural Commodity; RL32725; Library of Congress Washington DC Congressional Research Service: Washington, DC, USA, 2014. [Google Scholar]
- Kuglarz, M.; Alvarado-Morales, M.; Karakashev, D.; Angelidaki, I. Integrated production of cellulosic bioethanol and succinic acid from industrial hemp in a biorefinery concept. Bioresour. Technol. 2016, 200, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Yang, Y.; Pu, Y.; Meng, X.; Muchero, W.; Yee, K.L.; Thompson, O.A.; Rodriguez, M.; Bali, G.; Engle, N.L.; et al. Insights of biomass recalcitrance in natural Populus trichocarpa variants for biomass conversion. Green Chem. 2017, 19, 5467–5478. [Google Scholar] [CrossRef]
- Chheda, J.N.; Dumesic, J.A. An overview of dehydration, aldol-condensation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates. Catal. Today 2007, 123, 59–70. [Google Scholar] [CrossRef]
- Yoo, C.G.; Zhang, S.; Pan, X. Effective conversion of biomass into bromomethylfurfural, furfural, and depolymerized lignin in lithium bromide molten salt hydrate of a biphasic system. RSC Adv. 2017, 7, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Muensri, P.; Kunanopparat, T.; Menut, P.; Siriwattanayotin, S. Effect of lignin removal on the properties of coconut coir fiber/wheat gluten biocomposite. Composites Part A 2011, 42, 173–179. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Wei, J.; Guo, Q.; Ding, L.; Gong, Y.; Yu, J.; Yu, G. Understanding the effect of different biomass ash additions on pyrolysis product distribution, char physicochemical characteristics, and char gasification reactivity of bituminous coal. Energy Fuels 2019, 33, 3068–3076. [Google Scholar] [CrossRef]
- Jeong, K.; Jeong, H.J.; Lee, G.; Kim, S.H.; Kim, K.H.; Yoo, C.G. Catalytic effect of alkali and alkaline earth metals in lignin pyrolysis: A density functional theory study. Energy Fuels 2020, 34, 9734–9740. [Google Scholar] [CrossRef]
- Viswanathan, M.B.; Park, K.; Cheng, M.H.; Cahoon, E.B.; Dweikat, I.; Clemente, T.; Singh, V. Variability in structural carbohydrates, lipid composition, and cellulosic sugar production from industrial hemp varieties. Ind. Crops Prod. 2020, 157. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Wang, D. Conversion of liquid hot water, acid and alkali pretreated industrial hemp biomasses to bioethanol. Bioresour. Technol. 2020, 309, 123383. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Liu, E.; Saeed, A.; Williams, D.W.; Hu, H.; Li, C.; Ray, A.E.; Shi, J. Industrial hemp as a potential bioenergy crop in comparison with kenaf, switchgrass and biomass sorghum. Bioresour. Technol. 2017, 244, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, I.B.; Kuglarz, M.; Karakashev, D.; Angelidaki, I. Thermochemical pretreatments for enhancing succinic acid production from industrial hemp (Cannabis sativa L.). Bioresour. Technol. 2015, 182, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Wang, D. High Ethanol Concentration (77 g/L) of Industrial Hemp Biomass Achieved Through Optimizing the Relationship between Ethanol Yield/Concentration and Solid Loading. ACS Omega 2020, 5, 21913–21921. [Google Scholar] [CrossRef] [PubMed]
- Kuglarz, M.; Grübel, K. Integrated Production of Biofuels and Succinic Acid from Biomass after Thermochemical Pretreatments. Ecol. Chem. Eng. S 2018, 25, 521–536. [Google Scholar] [CrossRef] [Green Version]
- Moxley, G.; Zhu, Z.; Zhang, Y.-H.P. Efficient Sugar Release by the Cellulose Solvent-Based Lignocellulose Fractionation Technology and Enzymatic Cellulose Hydrolysis. J. Agric. Food Chem. 2008, 56, 7885–7890. [Google Scholar] [CrossRef]
- Gandolfi, S.; Ottolina, G.; Riva, S.; Fantoni, G.P.; Patel, I. Complete Chemical Analysis of Carmagnola Hemp Hurds and Structural Features of Its Components. BioResources 2013, 8, 2641–2656. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Garnaes, J.; Tunjic, S.; Mokkapati, V.R.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Gong, W.; Yang, Q.; Zhu, Z.; Yan, L.; Hu, Z.; Peng, Y. White-rot fungi pretreatment combined with alkaline/oxidative pretreatment to improve enzymatic saccharification of industrial hemp. Bioresour. Technol. 2017, 243, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kuglarz, M.; Gunnarsson, I.B.; Svensson, S.E.; Prade, T.; Johansson, E.; Angelidaki, I. Ethanol production from industrial hemp: Effect of combined dilute acid/steam pretreatment and economic aspects. Bioresour. Technol. 2014, 163, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Sporck, D.; Reinoso, F.A.M.; Rencoret, J.; Gutierrez, A.; Del Rio, J.C.; Ferraz, A.; Milagres, A.M.F. Xylan extraction from pretreated sugarcane bagasse using alkaline and enzymatic approaches. Biotechnol. Biofuels 2017, 10, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, C.G.; Nghiem, N.P.; Hicks, K.B.; Kim, T.H. Maximum production of fermentable sugars from barley straw using optimized soaking in aqueous ammonia (SAA) pretreatment. Appl. Biochem. Biotechnol. 2013, 169, 2430–2441. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.D.; Smith, T.J.; Li, N.; Zong, M.H. Novel renewable ionic liquids as highly effective solvents for pretreatment of rice straw biomass by selective removal of lignin. Biotechnol. Bioeng. 2012, 109, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Lee, C.W.; Kim, T.H. Two-stage fractionation of corn stover using aqueous ammonia and hot water. Appl. Biochem. Biotechnol. 2011, 164, 729–740. [Google Scholar] [CrossRef]
- Jiang, Y.; Lawrence, M.; Ansell, M.; Hussain, A. Cell wall microstructure, pore size distribution and absolute density of hemp shiv. R. Soc. Open Sci. 2018, 5, 171945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, M.; Wang, H.; Lau, K.; Cardona, F.; Aravinthan, T. Mechanical properties of chemically-treated hemp fibre reinforced sandwich composites. Composites Part B 2012, 43, 159–169. [Google Scholar] [CrossRef]
- Antony, S.; Cherouat, A.; Montay, G. Experimental, analytical and numerical analysis to investigate the tensile behaviour of hemp fibre yarns. Compos. Struct. 2018, 202, 482–490. [Google Scholar] [CrossRef]
- Shahzad, A. Hemp fiber and its composites—A review. J. Compos. Mater. 2012, 46, 973–986. [Google Scholar] [CrossRef]
- Jankauskienė, Z.; Butkutė, B.; Gruzdevienė, E.; Cesevičienė, J.; Fernando, A.L. Chemical composition and physical properties of dew-and water-retted hemp fibers. Ind. Crops Prod. 2015, 75, 206–211. [Google Scholar] [CrossRef]
- Shin, S.-J.; Han, S.-H.; Park, J.-M.; Cho, N.-S. Monosaccharides from industrial hemp (Cannabis sativa L.) woody core pretreatment with ammonium hydroxide soaking treatment followed by enzymatic saccharification. J. Korea TAPPI 2009, 41, 15–19. [Google Scholar]
- Stevulova, N.; Estokova, A.; Cigasova, J.; Schwarzova, I.; Kacik, F.; Geffert, A. Thermal degradation of natural and treated hemp hurds under air and nitrogen atmosphere. J. Therm. Anal. Calorim. 2017, 128, 1649–1660. [Google Scholar] [CrossRef] [Green Version]
- Sawpan, M.A.; Pickering, K.L.; Fernyhough, A. Effect of various chemical treatments on the fibre structure and tensile properties of industrial hemp fibres. Composites Part A 2011, 42, 888–895. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Sain, M.; Oksman, K. Study of structural morphology of hemp fiber from the micro to the nanoscale. Appl. Compos. Mater. 2007, 14, 89. [Google Scholar] [CrossRef]
- Li, X.; Du, G.; Wang, S.; Meng, Y. Influence of Gender on the Mechanical and Physical Properties of Hemp Shiv Fiber Cell Wall in Dioecious Hemp Plant. BioResources 2015, 10, 2281–2288. [Google Scholar] [CrossRef] [Green Version]
- Puangsin, B.; Soeta, H.; Saito, T.; Isogai, A. Characterization of cellulose nanofibrils prepared by direct TEMPO-mediated oxidation of hemp bast. Cellulose 2017, 24, 3767–3775. [Google Scholar] [CrossRef]
- Schwab, U.S.; Callaway, J.C.; Erkkilä, A.T.; Gynther, J.; Uusitupa, M.I.; Järvinen, T. Effects of hempseed and flaxseed oils on the profile of serum lipids, serum total and lipoprotein lipid concentrations and haemostatic factors. Eur. J. Nutr. 2006, 45, 470–477. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 277, 300–315. [Google Scholar] [CrossRef]
- Prade, T.; Svensson, S.-E.; Andersson, A.; Mattsson, J.E. Biomass and energy yield of industrial hemp grown for biogas and solid fuel. Biomass Bioenergy 2011, 35, 3040–3049. [Google Scholar] [CrossRef]
- Khattab, M.M.; Dahman, Y. Production and recovery of poly-3-hydroxybutyrate bioplastics using agro-industrial residues of hemp hurd biomass. Bioprocess Biosyst. Eng. 2019, 42, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Kamireddy, S.R.; Li, J.; Abbina, S.; Berti, M.; Tucker, M.; Ji, Y. Converting forage sorghum and sunn hemp into biofuels through dilute acid pretreatment. Ind. Crops Prod. 2013, 49, 598–609. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N.-O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Wawro, A.; Batog, J.; Gieparda, W. Chemical and enzymatic treatment of hemp biomass for bioethanol production. Appl. Sci. 2019, 9, 5348. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Xu, Y.; Zhang, M.; Wang, D. Integrating bran starch hydrolysates with alkaline pretreated soft wheat bran to boost sugar concentration. Bioresour. Technol. 2020, 302, 122826. [Google Scholar] [CrossRef]
- Kumar, R.; Hu, F.; Sannigrahi, P.; Jung, S.; Ragauskas, A.J.; Wyman, C.E. Carbohydrate derived-pseudo-lignin can retard cellulose biological conversion. Biotechnol. Bioeng. 2013, 110, 737–753. [Google Scholar] [CrossRef]
- Sipos, B.; Kreuger, E.; Svensson, S.-E.; Reczey, K.; Björnsson, L.; Zacchi, G. Steam pretreatment of dry and ensiled industrial hemp for ethanol production. Biomass Bioenergy 2010, 34, 1721–1731. [Google Scholar] [CrossRef] [Green Version]
- Kreuger, E.; Sipos, B.; Zacchi, G.; Svensson, S.-E.; Björnsson, L. Bioconversion of industrial hemp to ethanol and methane: The benefits of steam pretreatment and co-production. Bioresour. Technol. 2011, 102, 3457–3465. [Google Scholar] [CrossRef]
- Nykter, M.; Kymäläinen, H.-R.; Thomsen, A.B.; Lilholt, H.; Koponen, H.; Sjöberg, A.-M.; Thygesen, A. Effects of thermal and enzymatic treatments and harvesting time on the microbial quality and chemical composition of fibre hemp (Cannabis sativa L.). Biomass Bioenergy 2008, 32, 392–399. [Google Scholar] [CrossRef]
- Shin, S.-J.; Sung, Y.J. Improving enzymatic hydrolysis of industrial hemp (Cannabis sativa L.) by electron beam irradiation. Radiat. Phys. Chem. 2008, 77, 1034–1038. [Google Scholar] [CrossRef]
- Sung, Y.J.; Shin, S.-J. Compositional changes in industrial hemp biomass (Cannabis sativa L.) induced by electron beam irradiation Pretreatment. Biomass Bioenergy 2011, 35, 3267–3270. [Google Scholar] [CrossRef]
- Lam, N.D.; Nagasawa, N.; Kume, T. Effect of radiation and fungal treatment on lignocelluloses and their biological activity. Radiat. Phys. Chem. 2000, 59, 393–398. [Google Scholar] [CrossRef]
- Khan, F.; Ahmad, S.; Kronfli, E. γ-Radiation induced changes in the physical and chemical properties of lignocellulose. Biomacromolecules 2006, 7, 2303–2309. [Google Scholar] [CrossRef]
- Bouchard, J.; Methot, M.; Jordan, B. The effects of ionizing radiation on the cellulose of woodfree paper. Cellulose 2006, 13, 601–610. [Google Scholar] [CrossRef]
- Takacs, E.; Wojnarovits, L.; Földváry, C.; Hargittai, P.; Borsa, J.; Sajo, I. Effect of combined gamma-irradiation and alkali treatment on cotton–cellulose. Radiat. Phys. Chem. 2000, 57, 399–403. [Google Scholar] [CrossRef]
- Gandolfi, S.; Ottolina, G.; Consonni, R.; Riva, S.; Patel, I. Fractionation of hemp hurds by organosolv pretreatment and its effect on production of lignin and sugars. ChemSusChem 2014, 7, 1991–1999. [Google Scholar] [CrossRef]
- Barta, Z.; Oliva, J.; Ballesteros, I.; Dienes, D.; Ballesteros, M.; Réczey, K. Refining hemp hurds into fermentable sugars or ethanol. Chem. Biochem. Eng. Q. 2010, 24, 331–339. [Google Scholar]
- Wang, M.; Han, J.; Dunn, J.B.; Cai, H.; Elgowainy, A. Well-to-wheels energy use and greenhouse gas emissions of ethanol from corn, sugarcane and cellulosic biomass for US use. Environ. Res. Lett. 2012, 7, 045905. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Singh, V. Bioconversion of Processing Waste from Agro-Food Industries to Bioethanol: Creating a Sustainable and Circular Economy. In Waste Valorisation: Waste Streams in a Circular Economy; Wiley: Hoboken, NJ, USA, 2020; pp. 161–181. [Google Scholar] [CrossRef]
- Kricka, W.; James, T.C.; Fitzpatrick, J.; Bond, U. Engineering Saccharomyces pastorianus for the co-utilisation of xylose and cellulose from biomass. Microb. Cell Fact. 2015, 14, 61. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dien, B.S.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Fermentation of undetoxified sugarcane bagasse hydrolyzates using a two stage hydrothermal and mechanical refining pretreatment. Bioresour. Technol. 2018, 261, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Brazdausks, P.; Paze, A.; Rizhikovs, J.; Puke, M.; Meile, K.; Vedernikovs, N.; Tupciauskas, R.; Andzs, M. Effect of aluminium sulphate-catalysed hydrolysis process on furfural yield and cellulose degradation of Cannabis sativa L. shives. Biomass Bioenergy 2016, 89, 98–104. [Google Scholar] [CrossRef]
- Kumar, D.; Juneja, A.; Singh, V. Fermentation technology to improve productivity in dry grind corn process for bioethanol production. Fuel Process. Technol. 2018, 173, 66–74. [Google Scholar] [CrossRef]
- Mohagheghi, A.; Tucker, M.; Grohmann, K.; Wyman, C. High solids simultaneous saccharification and fermentation of pretreated wheat straw to ethanol. Appl. Biochem. Biotechnol. 1992, 33, 67–81. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Bilgen, S.; Sarıkaya, İ. Utilization of forestry and agricultural wastes. Energy Sources Part A 2016, 38, 3484–3490. [Google Scholar] [CrossRef]
- Pakarinen, A.; Maijala, P.; Stoddard, F.; Santanen, A.; Tuomainen, P.; Kymäläinen, M.; Viikari, L. Evaluation of annual bioenergy crops in the boreal zone for biogas and ethanol production. Biomass Bioenergy 2011, 35, 3071–3078. [Google Scholar] [CrossRef]
- Kreuger, E.; Prade, T.; Escobar, F.; Svensson, S.-E.; Englund, J.-E.; Björnsson, L. Anaerobic digestion of industrial hemp–Effect of harvest time on methane energy yield per hectare. Biomass Bioenergy 2011, 35, 893–900. [Google Scholar] [CrossRef]




| Biomass Samples | Cellulose [%] | Hemicellulose [%] | Lignin [%] | Ash [%] | Others [%] | Ref |
|---|---|---|---|---|---|---|
| Industrial hemp cultivars | 32.6–44.5 | 10.6–15.5 a | 17.0–21.5 | 2.6–7.6 | 5.3–20.0 (Extractives) | [24] |
| Industrial hemp | 36.5 | 17.0 a | 21.9 | - | 13.3 (Extractives) | [26] |
| 11.3 (Protein, ash) | ||||||
| Industrial hemp cultivars | 43.8–51.1 | 11.6–14.2 a | 15.4–29.4 | - | 3.7–11.9 (Extractives) | [13] |
| 0.3–23.1 (others) | ||||||
| Industrial hemp cultivars | 40.1–42.7 | 12.5–16.6 a | 14.6–17.8 | - | 11.8–17.7 (Extractives) | [25] |
| Industrial hemp (conventional vs. organic) | 39.8–42.0 | 15.4–15.7 | 13.2–15.0 | 4.7–5.8 | 3.1–3.8 (Protein) | [34] |
| 0.6–0.8 (Lipids) | ||||||
| Industrial hemp hurds | 42.4 | 28.0 | 17.5 b | - | - | [30] |
| Industrial hemp | 46.4 | 20.1 a | 15.0 | 2.4 | - | [16] |
| Industrial hemp | 42.3 | 18.2 | 22.9 | 4.2 | - | [27] |
| Industrial hemp fiber and shives | 42.9–57.5 | 5.1–20.4 | 16.2–23.9 | 0.0–2.9 | 0.6–0.8 (Formic acid) | [32] |
| 2.0–6.2 (Acetic acid) | ||||||
| 6.0–15.5 (Residuals) | ||||||
| Industrial hemp woody core | 37.3 | 19.8 | 12.4 | - | - | [33] |
| Industrial hemp | 40.7 | 13.3 a | 15.7 | - | 14.4 (Extractives) | [28] |
| Industrial hemp hurds | 75.0 (Holocellulose) | 23.0 | 1.2 | 1.1 (Oil-CH2Cl2) | [31] | |
| 0.8 (Oil-Acetone) | ||||||
| 44.0 (α-Cellulose) | 25.0 (Hemicellulose) | 0.6 (Pectin-Acidic water) | ||||
| 1.6 (Protein and amino acid-basic water) | ||||||
| Industrial hemp | 40.1 | 16.0 a | 14.8 | - | - | [29] |
| Biomass | Crystallinity | DP of Cellulose | Ref |
|---|---|---|---|
| Untreated and chemically modified hemp hurds | 35.7–49.2 | 585–1302 | [8] |
| 55.6–90.2 a | |||
| Tempo-oxidized hemp bast | - | 560–1100 b | [49] |
| Natural and treated hemp hurds | - | 200–1300 | [45] |
| Chemically treated industrial hemp fibers | 84.8–91.6 | - | [46] |
| Untreated and chemically treated hemp fibers | 57.4–71.2 | 1138–1155 b | [47] |
| Hemp Shiv Fiber | 38.8–50.1 | - | [48] |
| Feedstock | Pretreatment Condition | Hydrolysis Condition | Cellulose Conversion [%] | References | |
|---|---|---|---|---|---|
| Solid Loading [%] | Enzymes | ||||
| Industrial hemp (C. sativa L.) Fedora 17 strain | 7.5 | Celluclast 1.5 L® (20 FPU/g glucan) | Untreated biomass: 30.3 | [16] | |
| H2SO4 (1–1.5%), 180 °C (followed by steam pretreatment for 10 min) | H2SO4 pretreated biomass: 69.0–72.4 | ||||
| H2O2 (3%), 90 °C, 1–2 h | Novozyme 188 (15 IU/g glucan) | H2O2 pretreated biomass: 72.0–80.0 | |||
| 10% solids in all cases | |||||
| Five industrial hemp varieties: SC (Seward County), YC (York County), LC (Loup County), 19 m (19 m96136), and CBD (CBD Hemp) | Hydrothermal-mechanical refining: 180 °C, 10 min; 3 cycles of disk milling, 20% solids | 10.0 | Cellic® Ctec2 (16.95 mg cellulase protein/g dry substrate) | 62.3–85.8 | [24] |
| NS 22,244 (4.24 mg cellulase protein/g dry substrate) | |||||
| Industrial hemp (C. sativa L.) of Felina 32 variety | Acid-assisted steam pretreatment: H2SO4 (0–2%), 140/180 °C, 20/10 min, 10% solids | 5.0 | Celluclast 1.5 L® (30 FPU/g glucan) | Untreated biomass: 29.6 | [34] |
| Novozyme 188 (20 IU/g glucan) | Pretreated biomass: 37.7–73.6 | ||||
| Industrial hemp (C. sativa L.) of Uso 31 variety | 5.0 | Celluclast 1.5 L® (20 FPU/g glucan) | Untreated biomass: 22.8 | [27] | |
| H2SO4 (0–2%), 180 °C, 10 min | H2SO4 pretreated biomass: 48.0–73.9 | ||||
| NaOH (1–3%) 121 °C, 1 h | Novozyme 188 (15 IU/g glucan) | NaOH pretreated biomass: 78.0–80.1 | |||
| H2O2 (1–3%), 121 °C, 1 h | H2O2 pretreated biomass: 83.4–90.0 | ||||
| 10% solids in all cases | |||||
| Hemp hurds | Cellulose-solvent-based lignocellulose fractionation (CSLF) | 1.0 | Spezyme CP | Untreated biomass: 24.0 | [30] |
| cellulase (15 FPU/g glucan); Novozyme 188 (60 IU/g glucan) | Pretreated Biomass: 64.0–95.9 | ||||
| Five industrial hemp varieties: Helena, SS Beta, Tygra, and Eletta Campana | Liquid hot water, 170 °C, 30 min | 5.0 | Cellic® Ctec3 (30 FPU/g biomass) | LHW pretreated biomass: 53.9–71.7 | [25] |
| H2SO4 (1%), 170 °C, 30 min | H2SO4 pretreated biomass: 41.7–58.7 | ||||
| NaOH (1%) 170 °C, 30 min (1:10 solid-to-liquid ratio) | NS 22,244 (140 FXU/g biomass) | NaOH pretreated biomass: 59.1–88.9 | |||
| Hemp hurds (Cannabis sativa L.) | Steam pretreatment: | 5.0 | NS 50013 (15 FPU/g biomass) | [70] | |
| 220–230 °C, 10 min in 2 L reactor; | 2 L reactor: 80–82 | ||||
| 200–220 °C, 10 min in 10 L reactor | NS 50010 (volumetric ratio 0.1) | 10 L reactor: 62–83 | |||
| 11 different industrial hemp [6 fiber-only and 5 dual-purpose (fiber and grain)] | Acid Pretreatment: | 2.0 | CTec2 (20 mg protein/g biomass) | Untreated biomass: 14.7–29.8 | [13] |
| H2SO4 (1%), 160 °C, 30–50 min | HTec2 (10% v/v of CTec2) | Pretreated Biomass: 43.6–77.9 | |||
| Industrial hemp (C. sativa L.) of the variety Futura 75 (Dry and Ensiled) | Acid-assisted steam pretreatment | 2.0 | Celluclast 1.5 L® (15 FPU/g glucan); | [60] | |
| Dry hemp: 205–215 °C with 2% SO2 impregnation | Dry hemp: 72.7–87.6 | ||||
| Ensiled hemp: 190–220 °C, with 2% SO2 impregnation | Novozyme 188 (23 IU/g glucan) | Ensiled hemp: 58.3–89.3 | |||
| Materials | Pretreatment | SHF/SSF * | Microorganism | Solid Loading [%] | Ethanol Yield | Reference |
|---|---|---|---|---|---|---|
| Four industrial hemp varieties: Helena, SS Beta, Tygra, and Eletta Campana | Liquid hot water | SSF | S. cerevisiae | 5 | LHW pretreated biomass: 9.20–10.90 g/L | [25] |
| acid (H2SO4) | H2SO4 pretreated biomass: 11.94–13.77 g/L | |||||
| alkali (NaOH) | NaOH pretreated biomass: 18.21–20.29 g/L | |||||
| Industrial hemp (Tygra) | Alkali (NaOH) | SSF | S. cerevisiae | 6–21 | 25.1–65.9 g/L | [28] |
| Industrial hemp (C. sativa L.) of Felina 32 variety | Acid-assisted steam pretreatment | SHF | S. cerevisiae (3% v/v) | - | Untreated biomass: 2.89 g/L | [34] |
| Pretreated biomass: 4.62–10.00 g/L | ||||||
| Industrial hemp (C. sativa L.) of the variety Futura 75 (Dry and Ensiled) | Acid-assisted steam pretreatment | SSF | S. cerevisiae (5 g/L) | 7.5 | Dry hemp: 18.4–21.3 g/L | [60] |
| Ensiled hemp: 15.4–20.3 g/L | ||||||
| Industrial hemp (Fedora 17 strain) | Acid (H2SO4) followed by steam; alkaline oxidative (H2O2) | SHF | S. cerevisiae | 5 (during hydrolysis) | Untreated biomass: 7.2 ** | [16] |
| H2SO4 pretreated biomass: 14.9–15.5 ** | ||||||
| H2O2 pretreated biomass: 16.6–17.5 ** | ||||||
| Hemp hurds | Steam | SSF | S. cerevisiae 4% (v/w) | 10 | 8.5–14.1 ** | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, A.; Jia, L.; Kumar, D.; Yoo, C.G. Recent Advancements in Biological Conversion of Industrial Hemp for Biofuel and Value-Added Products. Fermentation 2021, 7, 6. https://doi.org/10.3390/fermentation7010006
Ji A, Jia L, Kumar D, Yoo CG. Recent Advancements in Biological Conversion of Industrial Hemp for Biofuel and Value-Added Products. Fermentation. 2021; 7(1):6. https://doi.org/10.3390/fermentation7010006
Chicago/Turabian StyleJi, Anqi, Linjing Jia, Deepak Kumar, and Chang Geun Yoo. 2021. "Recent Advancements in Biological Conversion of Industrial Hemp for Biofuel and Value-Added Products" Fermentation 7, no. 1: 6. https://doi.org/10.3390/fermentation7010006
APA StyleJi, A., Jia, L., Kumar, D., & Yoo, C. G. (2021). Recent Advancements in Biological Conversion of Industrial Hemp for Biofuel and Value-Added Products. Fermentation, 7(1), 6. https://doi.org/10.3390/fermentation7010006