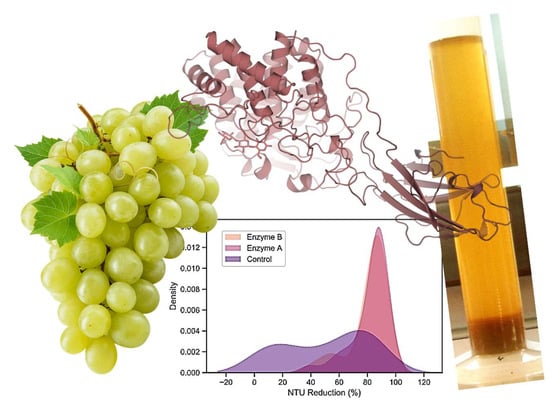
Addressing Enzymatic Clarification Challenges of Muscat Grape Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Clarification Tests
2.3. Juice Characterization
2.4. Statistical Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singleton, V.L.; Trousdale, E. White wine phenolics: Varietal and processing differences as shown by HPLC. Am. J. Enol. Vitic. 1983, 34, 27–34. [Google Scholar]
- Lukić, I.; Lotti, C.; Vrhovsek, U. Evolution of free and bound volatile aroma compounds and phenols during fermentation of Muscat blanc grape juice with and without skins. Food Chem. 2017, 232, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Horvat, I.; Radeka, S.; Damijanić, K.; Staver, M. Effect of different levels of skin disruption and contact with oxygen during grape processing on phenols, volatile aromas, and sensory characteristics of white wine. J. Food Process. Preserv. 2019, 43, e13969. [Google Scholar] [CrossRef]
- Ough, C.S.; Crowell, E.A. Use of Sulfur Dioxide in Winemaking. J. Food Sci. 1987, 52, 386–388. [Google Scholar] [CrossRef]
- Claus, H.; Mojsov, K. Enzymes for Wine Fermentation: Current and Perspective Applications. Fermentation 2018, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Garcia, A.; Carcel, C.; Dulau, L.; Samson, A.; Aguera, E.; Agosin, E.; Günata, Z. Influence of a Mixed Culture with Debaryomyces vanriji and Saccharomyces cerevisiae on the Volatiles of a Muscat Wine. J. Food Sci. 2002, 67, 1138–1143. [Google Scholar] [CrossRef]
- Karagiannis, S.; Lanaridis, P. Insoluble Grape Material Present in Must Affects the Overall Fermentation Aroma of Dry White Wines Made from Three Grape Cultivars Cultivated in Greece. J. Food Sci. 2002, 67, 369–374. [Google Scholar] [CrossRef]
- Ma, T.-Z.; Gong, P.-F.; Lu, R.-R.; Zhang, B.; Morata, A.; Han, S.-Y. Effect of Different Clarification Treatments on the Volatile Composition and Aromatic Attributes of ‘Italian Riesling’ Icewine. Molecules 2020, 25, 2657. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Smith, P.A. Current state of knowledge and challenges in wine clarification. Aust. J. Grape Wine Res. 2015, 21, 615–626. [Google Scholar] [CrossRef]
- Moio, L.; Ugliano, M.; Gambuti, A.; Genovese, A.; Piombino, P. Influence of Clarification Treatment on Concentrations of Selected Free Varietal Aroma Compounds and Glycoconjugates in Falanghina Vitis vinifera L. Must and Wine. Am. J. Enol. Vitic. 2004, 55, 7–12. [Google Scholar]
- Sharma, H.P.; Patel, H.; Sugandha. Enzymatic added extraction and clarification of fruit juices–A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1215–1227. [Google Scholar] [CrossRef]
- van Rensberg, P.; Pretorius, I.S. Enzymes in Winemaking: Harnessing Natural Catalysts for Efficient Biotransf ormations—A Review. South Afr. J. Enol. Vitic. 2019, 21, 52–73. [Google Scholar] [CrossRef]
- Martinez-Lapuente, L.; Guadalupe, Z.; Ayesterán, B. Properties of Wine Polysaccharides. In Pectins-Extraction, Purification, Characterization and Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Tapre, A.; Jain, R. Pectinases: Enzymes for fruit processing industry. Int. Food Res. J. 2014, 21, 447–453. [Google Scholar]
- Francioli, S.; Buxaderas, S.; Pellerin, P. Influence of Botrytis cinerea on the Polysaccharide Composition of Xarel.lo Musts and Cava Base Wines. Am. J. Enol. Vitic. 1999, 50, 456–460. [Google Scholar]
- Hettiarachchy, N.S.; Feliz, D.J.; Edwards, J.S.; Horax, R. 21—The use of immobilized enzymes to improve functionality. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 569–597. [Google Scholar] [CrossRef]
- Laffort. Protocol for Pectin Detection Using Pectin Test. Available online: https://laffort.com/wp-content/uploads/Autres/MODOP_EN_Pectintest.pdf (accessed on 17 August 2021).
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Möbius, C.H.; Görtges, S. Polyphenolbestimmung für die Praxis. Weinwissenschaft 1974, 29, 241–253. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wu, S. Glutathione suppresses the enzymatic and non-enzymatic browning in grape juice. Food Chem. 2014, 160, 8–10. [Google Scholar] [CrossRef]
- Jones-Moore, H.; Jelley, R.E.; Marangon, M.; Fedrizzi, B. The polysaccharides of winemaking: From grape to wine. Trends Food Sci. Technol. 2021, 111, 731–740. [Google Scholar] [CrossRef]
- Ducasse, M.-A.; Canal-Llauberes, R.-M.; de Lumley, M.; Williams, P.; Souquet, J.-M.; Fulcrand, H.; Doco, T.; Cheynier, V. Effect of macerating enzyme treatment on the polyphenol and polysaccharide composition of red wines. Food Chem. 2010, 118, 369–376. [Google Scholar] [CrossRef]
- Mojsov, K.; Ziberoski, J.; Božinović, Z.; Petreska, M. Comparison of effects of three commercial pectolytic enzyme preparations in white winemaking. Appl. Technol. Innov. 2011, 4, 34–38. [Google Scholar] [CrossRef]
- Rees, D.A. Structure, Conformation, and Mechanism in the Formation of Polysaccharide Gels and Networks. In Advances in Carbohydrate Chemistry and Biochemistry; Wolfrom, M.L., Tipson, R.S., Horton, D., Eds.; Academic Press: Cambridge, MA, USA, 1969; Volume 24, pp. 267–332. [Google Scholar]
- Vicens, A.; Fournand, D.; Williams, P.; Sidhoum, L.; Moutounet, M.; Doco, T. Changes in Polysaccharide and Protein Composition of Cell Walls in Grape Berry Skin (Cv. Shiraz) during Ripening and Over-Ripening. J. Agric. Food Chem. 2009, 57, 2955–2960. [Google Scholar] [CrossRef] [PubMed]
- Gregan, S.M.; Wargent, J.J.; Liu, L.; Shinkle, J.; Hofmann, R.; Winefield, C.; Trought, M.; Jordan, B. Effects of solar ultraviolet radiation and canopy manipulation on the biochemical composition of Sauvignon Blanc grapes. Aust. J. Grape Wine Res. 2012, 18, 227–238. [Google Scholar] [CrossRef]





| Cultivar | Condition | Pick | Enzyme Efficacy Index | Total Soluble Solids | Titratable Acidity | pH | Yeast Assimilable Nitrogen | Volatile Acidity | Gluconic Acid | A420 | Total Polyphenols FolinC | Total Proteins Bradford Assay | Ethanol-Insoluble Polysaccharides | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | EnzymeA | EnzymeB | (°brix) | (g/L) | (mg/L) | (g/L) | (g/L) | (AU) | (mg/L) | (mg/L) | (g/L) | ||||
| Orange | pasteurized | Machine | 0.14 | 0.25 | 0.17 | 26.7 | 2.8 | 3.81 | 292 | 0.24 | 0.3 | 0.230 | 383 | 534 | 0.00 |
| Orange | unpasteurized | Machine | 0.15 | 0.39 | 0.41 | 27.2 | 2.5 | 3.83 | 298 | 0.22 | 0.4 | 0.563 | 349 | 552 | 0.00 |
| Canelli | pasteurized | Machine | 0.14 | 0.14 | 0.17 | 21.6 | 4.4 | 3.46 | 136 | 0.30 | 0.3 | 0.126 | 633 | 529 | 5.56 |
| Canelli | unpasteurized | Machine | 0.62 | 0.55 | 0.56 | 21.2 | 4.1 | 3.45 | 135 | 0.28 | 0.4 | 0.475 | 469 | 564 | 4.13 |
| Canelli | pasteurized | Machine | 0.69 | 0.61 | 0.57 | 21.6 | 4.6 | 3.43 | 128 | 0.41 | 0.7 | 0.145 | 523 | 521 | 4.33 |
| Canelli | unpasteurized | Machine | 0.63 | 0.69 | 0.50 | 21.6 | 4.7 | 3.41 | 132 | 0.40 | 0.6 | 0.537 | 373 | 539 | 4.61 |
| Canelli | pasteurized | Machine | 0.66 | 0.92 | 0.93 | 24.6 | 2.3 | 3.58 | 133 | 0.10 | 0.0 | 0.058 | 323 | 471 | 7.17 |
| Canelli | unpasteurized | Machine | 0.20 | 0.92 | 0.91 | 24.2 | 2.0 | 3.58 | 132 | 0.10 | 0.1 | 0.388 | 311 | 678 | 3.65 |
| Canelli | pasteurized | Machine | 0.89 | 0.87 | 0.84 | 23.4 | 3.3 | 3.60 | 200 | 0.11 | 0.3 | 0.125 | 297 | 683 | 0.00 |
| Canelli | pasteurized | Machine | 0.85 | 0.87 | 0.86 | 24.1 | 3.5 | 3.61 | 215 | 0.13 | 0.2 | 0.083 | 377 | 489 | 5.59 |
| Canelli | unpasteurized | Machine | 0.72 | 0.91 | 0.91 | 23.8 | 3.0 | 3.61 | 227 | 0.13 | 0.4 | 0.233 | 305 | 542 | 0.00 |
| Canelli | unpasteurized | Machine | 0.61 | 0.91 | 0.90 | 23.0 | 3.0 | 3.60 | 216 | 0.11 | 0.3 | 0.185 | 306 | 514 | 0.00 |
| Canelli | pasteurized | Machine | 0.25 | 0.86 | 0.86 | 22.3 | 3.8 | 3.42 | 162 | 0.09 | 0.1 | 0.158 | 165 | 496 | 6.46 |
| Canelli | unpasteurized | Machine | 0.11 | 0.90 | 0.90 | 22.1 | 3.7 | 3.41 | 161 | 0.08 | 0.1 | 0.546 | 203 | 554 | 7.92 |
| Orange | pasteurized | Hand | 0.38 | 0.39 | 0.38 | 22.9 | 2.5 | 3.72 | 270 | 0.08 | 0.1 | 0.145 | 318 | 562 | 3.11 |
| Orange | unpasteurized | Hand | 0.76 | 0.87 | 0.85 | 22.2 | 2.1 | 3.72 | 271 | 0.09 | 0.2 | 0.553 | 243 | 595 | 0.00 |
| Orange | pasteurized | Hand | 0.34 | 0.42 | 0.58 | 23.9 | 2.7 | 3.79 | 313 | 0.10 | 0.2 | 0.190 | 367 | 579 | 2.36 |
| Orange | unpasteurized | Hand | 0.81 | 0.82 | 0.85 | 23.5 | 2.4 | 3.79 | 320 | 0.11 | 0.3 | 0.616 | 257 | 589 | 0.00 |
| Alexandria | pasteurized | Machine | 0.69 | 0.73 | 0.77 | 17.0 | 1.1 | 3.77 | 114 | 0.07 | 0.0 | 0.217 | 235 | 380 | 1.76 |
| Alexandria | unpasteurized | Machine | 0.23 | 0.60 | 0.58 | 16.8 | 1.1 | 3.78 | 117 | 0.10 | 0.0 | 0.406 | 230 | 479 | 1.52 |
| Golden | pasteurized | Hand | 0.87 | 0.70 | 0.89 | 18.7 | 1.9 | 3.69 | 231 | 0.02 | 0.0 | 0.060 | 269 | 428 | 2.02 |
| Golden | unpasteurized | Hand | 0.92 | 0.95 | 0.95 | 18.4 | 1.8 | 3.68 | 230 | 0.02 | 0.0 | 0.101 | 250 | 436 | 1.89 |
| Orange | pasteurized | Hand | 0.84 | 0.87 | 0.88 | 22.6 | 2.5 | 3.76 | 284 | 0.01 | 0.0 | 0.108 | 321 | 375 | 3.64 |
| Orange | unpasteurized | Hand | 0.74 | 0.90 | 0.91 | 22.3 | 2.3 | 3.76 | 279 | 0.02 | 0.1 | 0.362 | 233 | 428 | 0.00 |
| Canelli | pasteurized | Machine | 0.32 | 0.90 | 0.93 | 24.6 | 3.6 | 3.42 | 168 | 0.13 | −0.1 | 0.180 | 433 | 368 | 2.57 |
| Orange | pasteurized | Machine | 0.81 | 0.86 | 0.85 | 24.2 | 2.8 | 3.69 | 259 | 0.10 | 0.0 | 0.251 | 325 | 388 | 2.75 |
| Canelli | unpasteurized | Machine | 0.08 | 0.88 | 0.86 | 24.2 | 3.2 | 3.42 | 171 | 0.14 | 0.0 | 0.630 | 347 | 431 | 4.49 |
| Orange | unpasteurized | Machine | 0.33 | 0.88 | 0.88 | 23.8 | 2.3 | 3.71 | 276 | 0.09 | 0.1 | 0.388 | 300 | 469 | 6.08 |
| Alexandria | pasteurized | Machine | 0.91 | 0.86 | 0.89 | 21.8 | 1.2 | 3.99 | 145 | 0.06 | 0.0 | 0.388 | 237 | 456 | 3.77 |
| Alexandria | unpasteurized | Machine | 0.24 | 0.81 | 0.77 | 20.4 | 1.2 | 3.96 | 144 | 0.05 | 0.0 | 0.401 | 263 | 519 | 3.08 |
| Canelli | pasteurized | Machine | 0.83 | 0.88 | 0.91 | 25.2 | 3.1 | 3.48 | 149 | 0.11 | 0.1 | 0.175 | 410 | 378 | 4.72 |
| Canelli | unpasteurized | Machine | 0.51 | 0.90 | 0.91 | 24.7 | 2.8 | 3.49 | 153 | 0.10 | 0.3 | 0.374 | 387 | 441 | 2.99 |
| Alexandria | pasteurized | Machine | 0.88 | 0.86 | 0.87 | 19.4 | 1.9 | 3.74 | 131 | 0.15 | 0.4 | 0.281 | 240 | 388 | 2.34 |
| Alexandria | pasteurized | Machine | 0.80 | 0.84 | 0.68 | 19.8 | 1.8 | 3.73 | 132 | 0.14 | 0.3 | 0.216 | 237 | 365 | 2.75 |
| Alexandria | unpasteurized | Machine | 0.77 | 0.89 | 0.88 | 19.3 | 2.0 | 3.73 | 140 | 0.15 | 0.4 | 0.340 | 245 | 448 | 0.00 |
| Alexandria | unpasteurized | Machine | 0.50 | 0.90 | 0.90 | 19.7 | 1.9 | 3.71 | 132 | 0.14 | 0.3 | 0.316 | 229 | 416 | 0.00 |
| Alexandria | pasteurized | Machine | 0.53 | 0.51 | 0.55 | 20.2 | 1.9 | 3.76 | 135 | 0.22 | 0.6 | 0.240 | 277 | 363 | 0.00 |
| Alexandria | pasteurized | Machine | 0.59 | 0.57 | 0.61 | 21.1 | 1.8 | 3.75 | 136 | 0.19 | 0.5 | 0.226 | 278 | 365 | 0.00 |
| Alexandria | unpasteurized | Machine | 0.77 | 0.80 | 0.80 | 20.1 | 1.9 | 3.75 | 135 | 0.20 | 0.6 | 0.381 | 272 | 443 | 0.00 |
| Alexandria | unpasteurized | Machine | 0.84 | 0.89 | 0.83 | 20.9 | 1.9 | 3.74 | 137 | 0.17 | 0.5 | 0.408 | 252 | 438 | 0.00 |
| Alexandria | pasteurized | Machine | 0.90 | 0.55 | 0.90 | 17.3 | 2.4 | 3.76 | 202 | 0.03 | −0.1 | 0.311 | 211 | 448 | 1.93 |
| Alexandria | unpasteurized | Machine | 0.04 | 0.87 | 0.87 | 16.9 | 2.3 | 3.75 | 210 | 0.03 | 0.0 | 0.411 | 222 | 511 | 0.00 |
| Alexandria | pasteurized | Machine | 0.92 | 0.93 | 0.96 | 19.0 | 1.8 | 3.89 | 201 | 0.01 | −0.2 | 0.466 | 273 | 469 | 1.69 |
| Alexandria | unpasteurized | Machine | 0.04 | 0.94 | 0.95 | 18.7 | 1.4 | 3.90 | 210 | 0.01 | −0.1 | 0.539 | 279 | 537 | 0.00 |
| Orange | pasteurized | Hand | 0.02 | 0.04 | 0.04 | 26.4 | 1.3 | 4.00 | 210 | 0.03 | 0.0 | 0.448 | 341 | 411 | 3.52 |
| Orange | unpasteurized | Hand | 0.04 | 0.81 | 0.80 | 26.2 | 1.3 | 3.94 | 204 | 0.04 | 0.0 | 0.530 | 278 | 461 | 0.00 |
| Alexandria | pasteurized | Machine | 0.81 | 0.83 | 0.89 | 20.4 | 2.3 | 3.80 | 162 | 0.09 | −0.1 | 0.409 | 254 | 365 | 2.59 |
| Alexandria | pasteurized | Machine | 0.20 | 0.17 | 0.26 | 20.4 | 2.3 | 3.80 | 169 | 0.08 | −0.2 | 0.373 | 263 | 363 | 2.56 |
| Alexandria | unpasteurized | Machine | 0.44 | 0.85 | 0.83 | 20.1 | 2.0 | 3.80 | 176 | 0.07 | −0.1 | 0.428 | 292 | 469 | 0.00 |
| Alexandria | unpasteurized | Machine | 0.29 | 0.66 | 0.71 | 20.1 | 2.0 | 3.80 | 171 | 0.07 | −0.1 | 0.437 | 249 | 443 | 0.00 |
| Canelli | pasteurized | Machine | 0.80 | 0.69 | 0.79 | 25.9 | 2.2 | 3.57 | 92 | 0.11 | 0.1 | 0.177 | 384 | 534 | 3.37 |
| Canelli | unpasteurized | Machine | 0.82 | 0.90 | 0.90 | 25.6 | 1.8 | 3.57 | 104 | 0.10 | 0.2 | 0.198 | 347 | 552 | 4.21 |
| Alexandria | pasteurized | Machine | 0.40 | 0.38 | 0.32 | 23.9 | 1.9 | 3.84 | 151 | 0.11 | 0.4 | 0.290 | 369 | 512 | 0.00 |
| Alexandria | pasteurized | Machine | 0.11 | 0.19 | 0.36 | 23.6 | 1.8 | 3.83 | 154 | 0.12 | 0.3 | 0.252 | 385 | 498 | 0.00 |
| Alexandria | unpasteurized | Machine | 0.81 | 0.84 | 0.81 | 23.6 | 1.4 | 3.86 | 163 | 0.10 | 0.5 | 0.369 | 348 | 567 | 0.00 |
| Alexandria | unpasteurized | Machine | 0.81 | 0.84 | 0.84 | 23.3 | 1.5 | 3.85 | 168 | 0.09 | 0.4 | 0.369 | 358 | 526 | 0.00 |
| Alexandria | pasteurized | Machine | 0.84 | 0.80 | 0.83 | 23.1 | 1.9 | 3.87 | 152 | 0.10 | 0.7 | 0.113 | 578 | 325 | 0.00 |
| Alexandria | pasteurized | Machine | 0.68 | 0.79 | 0.78 | 22.0 | 1.9 | 4.00 | 172 | 0.10 | 0.2 | 0.327 | 519 | 303 | 0.00 |
| Alexandria | unpasteurized | Machine | 0.87 | 0.87 | 0.87 | 22.7 | 1.7 | 3.87 | 157 | 0.08 | 0.9 | 0.121 | 500 | 411 | 0.00 |
| Alexandria | unpasteurized | Machine | 0.55 | 0.73 | 0.79 | 21.8 | 1.7 | 4.00 | 183 | 0.11 | 0.4 | 0.409 | 633 | 427 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ridge, M.; Sommer, S.; Dycus, D.A. Addressing Enzymatic Clarification Challenges of Muscat Grape Juice. Fermentation 2021, 7, 198. https://doi.org/10.3390/fermentation7030198
Ridge M, Sommer S, Dycus DA. Addressing Enzymatic Clarification Challenges of Muscat Grape Juice. Fermentation. 2021; 7(3):198. https://doi.org/10.3390/fermentation7030198
Chicago/Turabian StyleRidge, Matt, Stephan Sommer, and Daniel A. Dycus. 2021. "Addressing Enzymatic Clarification Challenges of Muscat Grape Juice" Fermentation 7, no. 3: 198. https://doi.org/10.3390/fermentation7030198
APA StyleRidge, M., Sommer, S., & Dycus, D. A. (2021). Addressing Enzymatic Clarification Challenges of Muscat Grape Juice. Fermentation, 7(3), 198. https://doi.org/10.3390/fermentation7030198