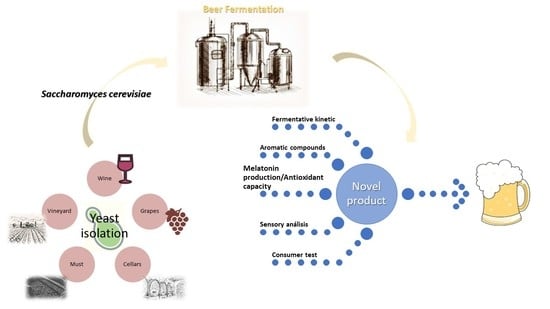
Wine Saccharomyces Yeasts for Beer Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Fermentation Screening
2.2.1. Lab Scale Fermentations
- Type I (white/creme): low-null production H2S
- Type II (light brown): moderate production H2S
- Type III (brown): high production H2S
- Type IV (dark brown/black): very high production H2S
2.2.2. Brewery Scale Fermentations
2.3. Analytical Methods
2.3.1. Residual Sugars, Glycerol, and Ethanol Determination
2.3.2. Volatile Compounds by GC
2.3.3. Melatonin Production by HPLC
2.3.4. Determination of Antioxidant Activity
2.4. Sensory Analysis
2.5. Consumer Acceptability Test
2.6. Statistical Analysis
3. Results and Discussion
3.1. Screening of S. cerevisiae Strains at Lab Scale
- The content of fermentable sugars in wort depends on the raw ingredients (mostly barley malt) and the method used in wort preparation. The order in which yeasts metabolise the fermentable sugars is as follows, glucose, fructose, maltose and maltotriose, maltose being the most abundant in wort. Complete and timely conversion of all sugars by Saccharomyces strains is the purpose for brewers. In this context, not all species are able to consume the four sugars [4]. The order in which sugars are fermented, may be the answer for non-maltose fermenting strains. Some studies suggest that glucose could control the maltose metabolism, repressing the synthesis of maltose transporters and of the α-glucosidases (maltases) that hydrolyse this sugar inside the cell [30,31]. Furthermore, maltose transport is more strongly inhibited by glucose in some ale strains than in some lager strains [32,33]. This could be an explanation for the behaviour of twelve yeast strains found in the study that were not able to ferment maltose.
- A total 70.9% of the strains showed a moderate production of H2S (Type II), whereas 10.3% showed a high production, including commercial strain S-04 (Type III) and 18.8% a low-null production. H2S is a volatile compound mostly unwanted, as it is responsible for a “rotten-egg” smell, thus masking other desired aromas in beer [34]. Its concentration changes during the fermentation process due to the depletion of fermentable sugars, with a rapid decrease observed when the assimilation rate falls below 0.05 w/w% h−1. However it could also vary by yeast capture of H2S at the end of the fermentation in green beer [35]. For this reason, despite the fact that most of the yeast strains were within production Type II, these aromas were not found in the final beers.
- The yeast strains were able to ferment wort in 100 mL fermentation, but in lower levels than the Saccharomyces control strain, S-04.
3.2. Beer Analysis
3.3. Aroma Compound Production in 1 L
3.4. Melatonin Production
3.5. Determination of Antioxidant Activity
3.6. Principal Components Analysis (PCA)
3.7. Sensory Analysis
3.8. Industrial Scale Fermentation
3.9. Consumers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Aquilani, B.; Laureti, T.; Poponi, S.; Secondi, L. Beer choice and consumption determinants when craft beers are tasted: An exploratory study of consumer preferences. Food Qual. Prefer. 2015, 41, 214–224. [Google Scholar] [CrossRef]
- Moura-Nunes, N.; Brito, T.C.; da Fonseca, N.D.; de Aguiar, P.F.; Monteiro, M.; Perrone, D.; Torres, A.G. Phenolic compounds of Brazilian beers from different types and styles and application of chemometrics for modeling antioxidant capacity. Food Chem. 2016, 199, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marongiu, A.; Zara, G.; Legras, J.-L.; Del Caro, A.; Mascia, I.; Fadda, C.; Budroni, M. Novel starters for old processes: Use of Saccharomyces cerevisiae strains isolated from artisanal sourdough for craft beer production at a brewery scale. J. Ind. Microbiol. Biotechnol. 2015, 42, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Cubillos, F.A.; Gibson, B.; Grijalva-Vallejos, N.; Krogerus, K.; Nikulin, J. Bioprospecting for brewers: Exploiting natural diversity for naturally diverse beers. Yeast 2019, 36, 383–398. [Google Scholar] [CrossRef]
- Rossi, S.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G.I.F. Evaluation of Saccharomyces cerevisiae strains isolated from non-brewing environments in beer production. J. Inst. Brew. 2018, 124, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Pires, E.J.; Teixeira, J.; Brányik, T.; Vicente, A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodolo, E.J.; Kock, J.L.; Axcell, B.C.; Brooks, M. The yeast Saccharomyces cerevisiae—The main character in beer brewing. FEMS Yeast Res. 2008, 8, 1018–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fix, G. Principles of Brewing Science: A Study of Serious Brewing Issues; Brewers Publications: New York, NY, USA, 1999. [Google Scholar]
- Rossi, S.; Sileoni, V.; Perretti, G.I.F.; Marconi, O. Characterization of the volatile profiles of beer using headspace solid-phase microextraction and gas chromatography-mass spectrometry. J. Sci. Food Agric. 2013, 94, 919–928. [Google Scholar] [CrossRef]
- Meilgaard, M.C. Prediction of flavor differences between beers from their chemical composition. J. Agric. Food Chem. 1982, 30, 1009–1017. [Google Scholar] [CrossRef]
- Garofalo, C.; Osimani, A.; Milanović, V.; Taccari, M.; Aquilanti, L.; Clementi, F. The Occurrence of Beer Spoilage Lactic Acid Bacteria in Craft Beer Production. J. Food Sci. 2015, 80, M2845–M2852. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.D.; Moreno, H.; Calvo, J.R. Melatonin present in beer contributes to increase the levels of melatonin and antioxidant capacity of the human serum. Clin. Nutr. 2009, 28, 188–191. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Faria, J.D.A.F.; Cruz, A.G. Characterization of Brazilian lager and brown ale beers based on color, phenolic compounds, and antioxidant activity using chemometrics. J. Sci. Food Agric. 2010, 91, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Vaudano, E.; Garcia-Moruno, E. Discrimination of Saccharomyces cerevisiae wine strains using microsatellite multiplex PCR and band pattern analysis. Food Microbiol. 2008, 25, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.; Fell, W.J.; Boekhout, T.; Robert, V. Methods for isolation, phenotypic characterization and maintenance of yeasts. In The Yeasts; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 1. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value-added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef]
- Turgeon, Z.; Jurgens, R.C.; Sierocinski, T.; Brimacombe, C.A.; Jin, Y.; Swanson, J.M.; Husnik, J.I. De Novo Hybridization Approach Expands Diversity of Industrially Applicable Lager Yeast Strains with Unique Genome Composition. Tech. Q. 2021, 58, 106–116. [Google Scholar] [CrossRef]
- Ortega, C.; López, R.; Cacho, J.; Ferreira, V. Fast analysis of important wine volatile compounds: Development and validation of a new method based on gas chromatographic–flame ionisation detection analysis of dichloromethane microextracts. J. Chromatogr. A 2001, 923, 205–214. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Melatonin is synthesised by yeast during alcoholic fermentation in wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef]
- Kocadağlı, T.; Yılmaz, C.; Gökmen, V. Determination of melatonin and its isomer in foods by liquid chromatography tandem mass spectrometry. Food Chem. 2014, 153, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Hevia, D.; Mayo, J.C.; Tan, D.-X.; Garcia, A.R.; Sainz, R.M. Melatonin Enhances Photo-Oxidation of 2′,7′-Dichlorodihydrofluorescein by an Antioxidant Reaction That Renders N1-Acetyl-N2-Formyl-5-Methoxykynuramine (AFMK). PLoS ONE 2014, 9, e109257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Mazza, G. Simultaneous analysis of serotonin, melatonin, piceid and resveratrol in fruits using liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.; Fanjul-Bolado, P.; Titoiu, A.-M.; Porumb, R.; Epure, P. Progress in Electrochemical (Bio)Sensors for Monitoring Wine Production. Chemosensors 2019, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- European Brewery Convention. Analytica—EBC Section 13 Sensory Analysis Method 13.0; Fachverlag Hans Carl: Nürnberg, Germany, 2004. [Google Scholar]
- European Brewery Convention. Analytica—EBC Section 13 Sensory Analysis Method 13.4; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- European Brewery Convention. Analytica—EBC Section 13 Sensory Analysis Method 13.12; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- European Brewery Convention. Analytica—EBC Section 13 Sensory Analysis Method 13.10; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- Varela, P.; Ares, G. Sensory profiling, the blurred line between sensory and consumer science. A review of novel methods for product characterization. Food Res. Int. 2012, 48, 893–908. [Google Scholar] [CrossRef]
- Budroni, M.; Zara, G.; Ciani, M.; Comitini, F. Saccharomyces and Non-Saccharomyces Starter Yeasts. Brew. Technol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Klein, C.J.; Olsson, L.; Rønnow, B.; Mikkelsen, J.D.; Nielsen, J. Alleviation of glucose repression of maltose metabolism by MIG1 disruption in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1996, 62, 4441–4449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federoff, H.J.; Eccleshall, T.R.; Marmur, J. Carbon catabolite repression of maltase synthesis in Saccharomyces carlsbergensis. J. Bacteriol. 1983, 156, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crumplen, R.M.; Slaughter, J.C.; Stewart, G.G. Characteristics of maltose transporter activity in an ale and larger strain of the yeast Saccharomyces cerevisiae. Lett. Appl. Microbiol. 1996, 23, 448–452. [Google Scholar] [CrossRef]
- Rautio, J.; Londesborough, J. Maltose Transport by Brewer’s Yeasts in Brewer’s Wort. J. Inst. Brew. 2003, 109, 251–261. [Google Scholar] [CrossRef]
- Briggs, D.; Boulton, C.; Brookes, P.; Stevens, R. Brewing; CRC Press: Boca Raton, FL, USA, 2004; Volume 1. [Google Scholar]
- Oka, K.; Hayashi, T.; Matsumoto, N.; Yanase, H. Decrease in hydrogen sulfide content during the final stage of beer fermentation due to involvement of yeast and not carbon dioxide gas purging. J. Biosci. Bioeng. 2008, 106, 253–257. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Formation of 4-vinyl and 4-ethyl derivatives from hydroxycinnamic acids: Occurrence of volatile phenolic flavour compounds in beer and distribution of Pad1-activity among brewing yeasts. Food Chem. 2008, 107, 221–230. [Google Scholar] [CrossRef]
- Mertens, S.; Steensels, J.; Gallone, B.; Souffriau, B.; Malcorps, P.; Verstrepen, K.J. Rapid Screening Method for Phenolic Off-Flavor (POF) Production in Yeast. J. Am. Soc. Brew. Chem. 2017, 75, 318–323. [Google Scholar] [CrossRef]
- De Francesco, G.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Screening of new strains of Saccharomycodes ludwigii and Zygosaccharomyces rouxii to produce low-alcohol beer. J. Inst. Brew. 2015, 121, 113–121. [Google Scholar] [CrossRef]
- Hughes, E.D.; Baxter, P.S. Beer: Quality, Safety and Nutritional Aspects; Royal Society of Chemistry: Cambridge, UK, 2001. [Google Scholar]
- Langstaff, S.A.; Lewis, M.J. The mouthfeel of beer—A review. J. Inst. Brew. 1993, 99, 31–37. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Vanbeneden, N.; Daenen, L.; Delvaux, F.; Delvaux, F.R. Decrease of Aged Beer Aroma by the Reducing Activity of Brewing Yeast. J. Agric. Food Chem. 2010, 58, 3107–3115. [Google Scholar] [CrossRef]
- Hubmann, G.; Foulquié-Moreno, M.R.; Nevoigt, E.; Duitama, J.; Meurens, N.; Pais, T.M.; Mathé, L.; Saerens, S.; Nguyen, H.T.T.; Swinnen, S.; et al. Quantitative trait analysis of yeast biodiversity yields novel gene tools for metabolic engineering. Metab. Eng. 2013, 17, 68–81. [Google Scholar] [CrossRef]
- Langstaff, S.A.; Guinard, J.-X.; Lewis, M.J. Instrumental evaluation of the mouthfeel of beer and correlation with sensory evaluation. J. Inst. Brew. 1991, 97, 427–433. [Google Scholar] [CrossRef]
- Parker, W.E.; Richardson, P.J. The quantitative determination of glycerol in beer by gas-liquid chromatography. J. Inst. Brew. 1970, 76, 191–198. [Google Scholar] [CrossRef]
- Engan, S. Organoleptic threshold values of some organic acids in beer. J. Inst. Brew. 1974, 80, 162–163. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Maharaj, Y.R.; Pillay, B. Effects of fermentation temperature on the composition of beer volatile compounds, organoleptic quality and spent yeast density. Electron. J. Biotechnol. 2011, 14, 5. [Google Scholar] [CrossRef] [Green Version]
- Christensen, J.; Ladefoged, A.M.; Nørgaard, L. Rapid Determination of Bitterness in Beer Using Fluorescence Spectroscopy and Chemometrics. J. Inst. Brew. 2005, 111, 3–10. [Google Scholar] [CrossRef]
- Popescu, V.; Soceanu, A.; Dobrinas, S.; Stanciu, G. A study of beer bitterness loss during the various stages of the Romanian beer production process. J. Inst. Brew. 2013, 119, 111–115. [Google Scholar] [CrossRef]
- Bamforth, C.W. Beer and Health; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Laws, D.R.J.; McGuinness, J.D.; Rennie, H. The losses of bitter substances during fermentation. J. Inst. Brew. 1972, 78, 314–321. [Google Scholar] [CrossRef]
- Meilgaard, E.; Elizondo, M.; Moya, A. A study of carbonyl compounds in beer, part II. Flavor and flavor thresholds of aldehydes and ketones added to beer. Tec. Q. Master. Brew. Assac. Am. 1970, 7, 143–149. [Google Scholar]
- Guido, L.F. Sulfites in beer: Reviewing regulation, analysis and role. Sci. Agric. 2016, 73, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Meilgaard, M.C. Individual differences in sensory threshold for aroma chemicals added to beer. Food Qual. Prefer. 1993, 4, 153–167. [Google Scholar] [CrossRef]
- Dvořák, J.; Dostálek, P.; Štěrba, K.; Čejka, P.; Kellner, V.; Čulík, J.; Beinrohr, E. Determination of Total Sulphur Dioxide in Beer Samples by Flow-Through Chronopotentiometry. J. Inst. Brew. 2006, 112, 308–313. [Google Scholar] [CrossRef]
- Meilgaard, M. Flavor chemistry of beer: Part II: Flavor and threshold of 239 aroma volatiles. Master. Brew. Assoc. Am. Tech. Q. 1975, 12, 151–168. [Google Scholar]
- Kobayashi, M.; Shimizu, H.; Shioya, S. Beer Volatile Compounds and Their Application to Low-Malt Beer Fermentation. J. Biosci. Bioeng. 2008, 106, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Romano, P.; Suzzi, G.; Comi, G.; Zironi, R. Higher alcohol and acetic acid production by apiculate wine yeasts. J. Appl. Bacteriol. 1992, 73, 126–130. [Google Scholar] [CrossRef]
- Piddocke, M.P.; Kreisz, S.; Heldt-Hansen, H.P.; Nielsen, K.F.; Olsson, L. Physiological characterization of brewer’s yeast in high-gravity beer fermentations with glucose or maltose syrups as adjuncts. Appl. Microbiol. Biotechnol. 2009, 84, 453–464. [Google Scholar] [CrossRef]
- Meilgaard, M.C. Flavor chemistry in beer: Part I: Flavor interaction between principal volatiles. Master. Brew. Assoc. Am. Tech. Q. 1975, 12, 107–117. [Google Scholar]
- Clapperton, J.F.; Brown, D.G.W. Caprylic flavour as a feature of beer flavour. J. Inst. Brew. 1978, 84, 90–92. [Google Scholar] [CrossRef]
- Olšovská, J.; Vrzal, T.; Štěrba, K.; Slabý, M.; Kubizniaková, P.; Čejka, P. The chemical profiling of fatty acids during the brewing process. J. Sci. Food Agric. 2018, 99, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Reazin, G.; Scales, H.; Andreasen, A. Mechanism of major congener formation in alcoholic grain fermentations. J. Agric. Food Chem. 1970, 18, 585–589. [Google Scholar] [CrossRef]
- Gallone, B.; Steensels, J.; Prahl, T.; Soriaga, L.; Saels, V.; Herrera-Malaver, B.; Merlevede, A.; Roncoroni, M.; Voordeckers, K.; Miraglia, L.; et al. Domestication and Divergence of Saccharomyces cerevisiae Beer Yeasts. Cell 2016, 166, 1397–1410.e16. [Google Scholar] [CrossRef] [Green Version]
- Sterckx, F.L.; Missiaen, J.; Saison, D.; Delvaux, F.R. Contribution of monophenols to beer flavour based on flavour thresholds, interactions and recombination experiments. Food Chem. 2011, 126, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Moreno, H.; Calvo, J.R.; Maldonado, M.D. High levels of melatonin generated during the brewing process. J. Pineal Res. 2012, 55, 26–30. [Google Scholar] [CrossRef]
- Vigentini, I.; Gardana, C.; Fracassetti, D.; Gabrielli, M.; Foschino, R.; Simonetti, P.; Tirelli, A.; Iriti, M. Yeast contribution to melatonin, melatonin isomers and tryptophan ethyl ester during alcoholic fermentation of grape musts. J. Pineal Res. 2015, 58, 388–396. [Google Scholar] [CrossRef]
- Morcillo-Parra, M.; Beltran, G.; Mas, A.; Torija, M.-J. Effect of Several Nutrients and Environmental Conditions on Intracellular Melatonin Synthesis in Saccharomyces cerevisiae. Microorganisms 2020, 8, 853. [Google Scholar] [CrossRef]
- Tafulo, P.A.R.; Queirós, R.; Delerue-Matos, C.; Sales, M.G.F. Control and comparison of the antioxidant capacity of beers. Food Res. Int. 2010, 43, 1702–1709. [Google Scholar] [CrossRef] [Green Version]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef]
- Aliani, M.N.A.; Eskin, M. Bitterness; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Callejo, M.J.; Navas, J.J.G.; Alba, R.; Escott, C.; Loira, I.; González, M.C.; Morata, A. Wort fermentation and beer conditioning with selected non-Saccharomyces yeasts in craft beers. Eur. Food Res. Technol. 2019, 245, 1229–1238. [Google Scholar] [CrossRef]
- Jardim, C.D.C.; De Souza, D.; Machado, I.C.K.; Pinto, L.M.N.; Ramos, R.C.D.S.; Garavaglia, J. Sensory Profile, Consumer Preference and Chemical Composition of Craft Beers from Brazil. Beverages 2018, 4, 106. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, S.C.; De Castro, H.F.; Visconti, A.E.S.; Giudici, R. Scale-up effects on kinetic parameters and on predictions of a yeast recycle continuous ethanol fermentation model incorporating loss of cell viability. Bioprocess Biosyst. Eng. 2000, 23, 51–55. [Google Scholar] [CrossRef]
- Díaz, M.; García, A.I.; García, L.A. Mixing power, external convection, and effectiveness in bioreactors. Biotechnol. Bioeng. 1996, 51, 131–140. [Google Scholar] [CrossRef]
- Ahmad, M.; Holland, C.; McKay, G. Mass transfer studies in batch fermentation: Mixing characteristics. J. Food Eng. 1994, 23, 145–158. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Daenen, L.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Furfuryl Ethyl Ether: Important Aging Flavor and a New Marker for the Storage Conditions of Beer. J. Agric. Food Chem. 2004, 52, 1661–1668. [Google Scholar] [CrossRef]
- Shellhammer, T.H.; Bamforth, C.W. Assessing Color Quality of Beer. ACS Symp. Ser. 2008, 192–202. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.; Derdelinckx, G.; Verachtert, H. Bioflavoring and beer refermentation. Appl. Microbiol. Biotechnol. 2003, 62, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Eßlinger, H.M. Handbook of Brewing: Processes, Technology, Markets; Wiley: Weinheim, Germany, 2009. [Google Scholar]
- Calvo-Porral, C.; Rivaroli, S.; Orosa-Gonzalez, J. How consumer involvement influences beer flavour preferences. Int. J. Wine Bus. Res. 2020, 32, 537–554. [Google Scholar] [CrossRef]



| Yeast Strains | CO2 (g L−1) | Residual Fermentable Sugars (g L−1) | Apparent Attenuation (%) | Ethanol % (v/v) | Glycerol (g L−1) | |||
|---|---|---|---|---|---|---|---|---|
| Maltotriose | Maltose | Glucose | Fructose | |||||
| G 4 | 12.35 ± 1.06 f | 13.68 ± 0.04 a | 63.18 ± 0.02 a | 0.94 ± 0.01 b | 0.31 ± 0.00 a | 21.00 ± 0.01 f | 1.59 ± 0.02 e | 1.75 ± 0.01 e |
| G 354 | 51.55 ± 3.32 cd | 13.33 ± 2.24 abc | 3.75 ± 0.03 b | 0.70 ± 0.03 c | 0.17 ± 0.01 b | 72.00 ± 0.00 b | 5.07 ± 0.04 b | 3.63 ± 0.04 ab |
| G 450 | 51.05 ± 2.33 cd | 12.81 ± 0.08 cde | 3.40 ± 0.08 bc | 0.57 ± 0.00 cd | 0.17 ± 0.00 b | 68.90 ± 0.00 d | 4.63 ± 0.02 c | 3.10 ± 0.08 d |
| G 487 | 51.70 ± 0.14 cd | 12.48 ± 0.06 e | 3.45 ± 0.03 bc | 0.62 ± 0.01 c | 0.16 ± 0.00 b | 68.90 ± 0.00 d | 4.55 ± 0.02 c | 3.58 ± 0.15 abc |
| G 502 | 57.95 ± 0.35 b | 13.32 ± 0.01 abcd | 3.53 ± 0.03 bc | 0.66 ± 0.01 c | 0.18 ± 0.00 b | 72.00 ± 0.00 b | 5.10 ± 0.04 b | 3.68 ± 0.08 a |
| G 520 | 47.70 ± 0.99 de | 13.24 ± 0.01 abcd | 3.30 ± 0.00 cd | 0.57 ± 0.01 cd | 0.20 ± 0.01 b | 72.00 ± 0.00 b | 5.07 ± 0.05 b | 3.19 ± 0.14 cd |
| CLI 70 | 48.50 ±1.41 de | 12.73 ± 0.56 cde | 3.14 ± 0.38 cd | 0.40 ± 0.23 d | 0.19 ± 0.02 b | 68.90 ± 0.00 d | 4.43 ± 0.14 c | 3.26 ± 0.07 bcd |
| CLI 275 | 46.55 ± 0.49 de | 12.97 ± 0.01 bcde | 2.98 ± 0.03 d | 0.61 ± 0.01 c | 0.18 ± 0.00 b | 71.10 ± 0.03 c | 4.56 ± 0.01 c | 3.25 ± 0.02 bcd |
| CLI 1056 | 44.45 ± 2.47 e | 12.70 ± 0.31 de | 3.52 ±0.24 bc | 5.72 ± 0.05 a | 0.15 ± 0.06 b | 64.00 ± 0.00 e | 3.97 ± 0.16 d | 3.55 ± 0.35 abc |
| CLI 1109 | 55.50 ± 2.83 bc | 13.50 ± 0.12 ab | 3.43 ± 0.04 bc | 0.6 ± 0.00 c | 0.19 ± 0.01 b | 71.00 ± 0.01 c | 5.07 ± 0.03 b | 3.61 ± 0.10 ab |
| S-04 | 66.5 ± 0.85 a | 1.43 ± 0.08 f | 1.45 ± 0.01 e | 0.16 ± 0.00 e | 0.18 ± 0.02 b | 82.22 ± 0.00 a | 5.53 ± 0.05 a | 3.43 ± 0.01 abcd |
| Yeast Strains | Lactic Acid ppm | Colour EBC | Bitterness IBU | VDKs ppm | SO2 ppm |
|---|---|---|---|---|---|
| G 4 | 208.50 ± 3.50 c | 12.00 ± 0.00 | 34.40 ± 0.30 a | ≤0.05 | ≤1 |
| G 354 | 278.50 ± 21.50 ab | 11.00 ± 0.00 | 30.60 ± 2.80 ab | ≤0.05 | 1.90 ± 0.00 b |
| G 450 | 266.00 ± 7.00 abc | 11.00 ± 0.00 | 28.60 ± 2.00 abc | ≤0.05 | 2.05 ± 1.05 b |
| G 487 | 417.50 ± 12.50 a | 11.00 ± 0.00 | 32.30 ± 2.00 a | ≤0.05 | 1.70 ± 0.70 b |
| G 502 | 236.00 ± 13.00 bc | 11.00 ± 0.00 | 20.80 ± 0.50 de | ≤0.05 | ≤1 |
| G 520 | 321.00 ± 37.00 a | 13.00 ± 1.00 | 21.80 ± 1.50 de | 0.03 ± 0.03 bc | ≤1 |
| CLI 70 | 317.33 ± 21.46 a | 12.00 ± 1.00 | 23.13 ± 4.39 cde | 0.02 ± 0.03 bc | ≤1 |
| CLI 275 | 232.00 ± 21.00 bc | 11.50 ± 0.50 | 24.55 ± 3.25 bcd | 0.07 ± 0.01 ab | ≤1 |
| CLI 1056 | 259.30 ± 37.07 abc | 10.50 ± 0.50 | 17.30 ± 2.88 e | 0.03 ± 0.03 bc | ≤1 |
| CLI 1109 | 248.50 ± 9.50 bc | 12.00 ± 0.00 | 21.90 ± 0.70 de | ≤0.05 | 3.60 ± 0.10 a |
| S-04 | 321.00 ± 21.00 a | 13.00 ± 0.00 | 16.5 ± 0.30 e | 0.11 ± 0.03 a | ≤1 |
| Yeast Strains | G 4 | G 354 | G 450 | G 487 | G 502 | G 520 | CLI 70 | CLI 275 | CLI 1056 | CLI 1109 | S-04 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Higher alcohols | |||||||||||
| Isobutanol | 3.70 ± 0.31 e | 15.29 ± 0.14 b | 10.77 ± 1.76 cd | 14.26 ± 0.74 bc | 7.58 ± 0.50 de | 25.71 ± 0.90 a | 14.42 ± 1.87 bc | 11.17 ± 0.46 bcd | 23.78 ± 1.82 a | 24.68 ± 1.59 a | 23.54 ± 2.78 a |
| Isoamyl alcohol | 29.77 ± 3.66 f | 69.38 ± 0.58 b | 53.39 ± 1.38 de | 62.07 ± 1.58 bcd | 51.53 ± 8.59 e | 97.73 ± 2.11 a | 58.32 ± 0.75 cde | 63.80 ± 2.15 bcd | 70.81 ± 1.93 b | 88.41 ± 2.45 a | 68.73 ± 5.45 bc |
| Methionol | 0.41 ± 0.02 c | 0.05 ± 0.02 de | 0.13 ± 0.00 de | 0.09 ± 0.03 de | 0.86 ± 0.01 a | 0.79 ± 0.03 a | 0.02 ± 0.00 e | 0.61 ± 0.06 b | 0.09 ± 0.01 de | 0.17 ± 0.11 d | 0.82 ± 0.09 a |
| β-phenylethanol | 2.98 ± 0.37 i | 9.98 ± 0.98 h | 11.57 ± 0.11 gh | 20.91 ± 0.57 de | 16.39 ± 0.67 ef | 52.99 ± 1.56 a | 11.70 ± 0.99 fgh | 23.07 ± 0.25 d | 37.46 ± 2.58 c | 16.10 ± 0.06 fg | 45.91 ±4.09 b |
| Esters | |||||||||||
| Isoamyl acetate | 0.22 ± 0.01 e | 0.61± 0.08 de | 0.42 ± 0.17 e | 0.40 ± 0.19 e | 1.30 ± 0.10 c | 2.34 ± 0.06 a | 0.89 ± 0.17 cd | 0.65 ± 0.04 de | 0.40 ± 0.01 e | 1.90 ± 0.32 ab | 1.81 ± 0.26 b |
| Ethyl hexanoate | 0.09 ± 0.02 c | 0.01 ± 0.01 c | 0.16 ± 0.00 a | 0.09 ± 0.07 abc | 0.03 ± 0.01 c | 0.02 ± 0.00 c | 0.07 ± 0.06 abc | 0.01 ± 0.00 c | 0.15 ± 0.01 ab | 0.60 ± 0.03 bc | 0.03 ± 0.00 c |
| Ethyl octanoate | 0.13 ± 0.02 bcd | 0.15± 0.00 abcd | 0.08 ± 0.07 d | 0.11 ± 0.00 cd | 0.21 ± 0.00 a | 0.18 ± 0.00 ab | 0.15 ± 0.01 abcd | 0.16 ± 0.01 abc | 0.09 ± 0.01 cd | 0.21 ± 0.02 a | 0.13 ± 0.00 bcd |
| 2-phenylethyl acetate | 0.01 ± 0.00 d | 0.02 ± 0.00 d | 0.02 ± 0.01 bc | 0.02 ± 0.00 cd | 0.03 ± 0.00 ab | 0.03 ± 0.00 ab | 0.03 ± 0.00 ab | 0.03 ± 0.00 ab | 0.03 ± 0.00 ab | 0.03 ± 0.00 a | 0.01 ± 0.00 e |
| Fatty Acids | |||||||||||
| Isobutyric acid | nd | nd | 0.86 ± 0.01 b | 0.92 ± 0.02 b | nd | 0.88 ± 0.88 b | nd | 1.18 ± 0.00 ab | 1.72 ± 0.06 a | nd | nd |
| Butyric acid | nd | nd | 2.27 ± 0.15 a | 2.33 ± 0.04 a | nd | 2.35 ± 0.10 b | nd | 2.37 ± 0.05 a | 2.35 ± 0.02 a | nd | nd |
| Isovaleric acid | 3.24 ± 0.09 f | 6.09 ± 0.07 bc | 3.74 ± 0.06 ef | 3.81 ± 0.02 ef | 5.65 ± 0.17 bcd | 6.38 ± 0.13 b | 5.19 ± 0.04 cd | 5.95 ± 0.06 bc | 4.78 ± 0.21 de | 5.34 ± 1.07 bcd | 9.81 ± 0.59 a |
| Hexanoic acid | 0.81 ± 0.03 e | 2.31 ± 0.03 bc | 1.64 ± 0.14 d | nd | 2.57 ± 0.12 b | 2.68 ± 0.05 ab | 2.32 ± 0.44 bc | 1.90 ± 0.17 cd | 0.75 ± 0.01 e | 3.09 ± 0.22 a | 1.56 ± 0.07 d |
| Octanoic acid | 3.15 ± 0.18 f | 5.96 ± 0.01 cd | 5.71 ± 0.69 d | 4.18 ± 0.20 ef | 7.75 ± 0.19 ab | 8.63 ± 0.03 a | 7.15 ± 0.09 bc | 6.01 ± 0.36 cd | 4.22 ± 0.05 ef | 8.74 ± 0.67 a | 5.51 ± 0.35 de |
| Decanoic acid | 0.63 ± 0.06 bc | 0.38 ± 0.06 bc | 0.54 ± 0.16 bc | 0.45 ± 0.11 bc | 0.76 ± 0.40 bc | 2.57 ± 0.52 a | 0.42 ± 0.03 bc | 0.80 ± 0.00 bc | 0.55 ± 0.01 bc | 1.11 ± 0.74 b | 0.16 ± 0.02 c |
| Guaiacol | 0.06 ± 0.01 b | nd | 0.03 ± 0.03 bc | nd | 0.03 ± 0.03 bc | 0.05 ± 0.01 b | nd | nd | nd | 0.03 ± 0.03 bc | 0.13 ± 0.00 a |
| Yeast Strains | Melatonin (ng mL−1) | Ethanol % (v/v) |
|---|---|---|
| G 4 | 33.60 ± 9.57 b | 1.59 ± 0.02 e |
| G 354 | nd | 5.07 ± 0.04 b |
| G 450 | 30.14 ± 0.09 bc | 4.63 ± 0.02 c |
| G 487 | 25.98 ± 5.52 bc | 4.55 ± 0.02 c |
| G 502 | 5.04 ± 0.95 d | 5.10 ± 0.04 b |
| G 520 | 28.87 ± 1.50 bc | 5.07 ± 0.05 b |
| CLI 70 | 31.61 ± 2.59 bc | 4.43 ± 0.14 c |
| CLI 275 | nd | 4.56 ± 0.01 c |
| CLI 1056 | 56.51 ± 1.66 a | 3.97 ± 0.16 d |
| CLI 1109 | 27.41 ± 1.86 bc | 5.07 ± 0.03 b |
| S-04 | 20.41 ± 5.25 c | 5.53 ± 0.05 a |
| Yeast Strains | Q1 | Q2 | Qt |
|---|---|---|---|
| G 4 | 2.99 ± 0.20 e | 6.21 ± 0.37 b | 9.20 ± 0.57 d |
| G 354 | 3.89 ± 0.25 abcd | 9.14 ± 0.37 a | 13.03 ± 0.57 abc |
| G 450 | 3.93 ± 0.16 bcde | 8.60 ± 0.25 a | 11.64 ± 0.09 abc |
| G 487 | 3.39 ± 0.13 de | 8.25 ± 0.10 a | 11.64 ± 0.23 c |
| G 502 | 4.41 ± 0.28 abc | 9.04 ± 0.45 a | 13.48 ± 0.73 ab |
| G 520 | 4.74 ± 0.20 a | 8.68 ± 0.03 a | 13.43 ± 0.18 ab |
| CLI 70 | 3.84 ± 0.26 cde | 8.53 ± 0.37 a | 12.37 ± 0.24 abc |
| CLI 275 | 4.67 ± 0.59 ab | 8.80 ± 0.04 a | 13.67 ± 0.63 a |
| CLI 1056 | 4.31 ± 0.26 abc | 8.88 ± 0.47 a | 13.20 ± 0.63 ab |
| CLI 1109 | 4.07 ± 0.20 abcd | 8.19 ± 0.44 a | 12.26 ± 0.64 bc |
| S-04 | 3.78 ± 0.00 cde | 8.37 ± 0.21 a | 12.15 ± 0.21 bc |
| Parameters | 1 L Fermentation | 100 L Fermentation | ||
|---|---|---|---|---|
| G 520 | S-04 | G 520 | S-04 | |
| Ethanol (% v/v) | 5.07 ± 0.05 ab | 5.53 ± 0.05 a | 4.15 ± 0.23 c | 4.60 ± 0.43 bc |
| Glycerol (g L−1) | 3.19 ± 0.14 | 3.43 ± 0.01 | 3.11 ± 0.18 | 2.93 ± 0.46 |
| Lactic acid (ppm) | 321.00 ± 37.00 ab | 321.00 ± 21.00 ab | 268.50 ± 6.36 b | 337.00 ± 17.00 a |
| Colour (EBC) | 13 ± 1.00 a | 13 ± 0.00 a | 5.50 ± 0.71 b | 5.67 ± 1.15 b |
| Bitterness (IBU) | 21.80 ± 1.50 a | 16.50 ± 0.30 b | 22.35 ± 0.49 a | 23.40 ± 3.33 a |
| SO2 (ppm) | ≤1 b | ≤1 b | 1.15 ± 0.07 a | 1.07 ± 0.12 a |
| Total higher alcohols (mg L−1) | 177.29 ± 4.60 a | 139.08 ± 12.41 b | 91.11 ± 5.21 c | 80.41 ± 5.20 c |
| Total esters (mg L−1) | 3.06 ± 0.09 a | 2.89 ± 0.11 a | 0.76 ± 0.12 c | 1.12 ± 0.10 b |
| Total fatty acids (mg L−1) | 22.33 ± 1.49 a | 17.05 ± 1.03 b | 13.00 ± 0.18 c | 17.01 ± 0.66 b |
| γ-Butyrolactone (mg L−1) | 0.25 ± 0.00 a | 0.27 ± 0.00 a | 0.13 ± 0.02 b | 0.15 ± 0.04 b |
| Guaiacol (mg L−1) | 0.05 ± 0.01 b | 0.13 ± 0.00 a | nd | 0.07 ± 0.02 b |
| Melatonin (ng mL−1) | 28.87 ± 2.13 | 20.41 ± 5.25 | 22.04 ± 3.33 | 22.87 ± 3.07 |
| Antioxidant capacity (Qt) (mmol TE L−1) | 13.43 ± 0.18 a | 12.15 ± 0.21 ab | 12.06 ± 2.15 ab | 10.05 ± 0.52 b |
| Sensory Attributes | Response (n = 112) |
|---|---|
| Appearance | |
| Foam consistency | |
| Light | 54 |
| Fine | 29 |
| Medium | 21 |
| Persistent | 4 |
| Creamy | 4 |
| Visual impression | |
| Very haze | 19 |
| Hazy | 49 |
| Dull | 10 |
| Clear | 24 |
| Bright | 10 |
| Aroma | |
| Aroma intensity | |
| Low | 3 |
| Low-medium | 13 |
| Medium | 31 |
| Medium-high | 51 |
| High | 14 |
| Taste | |
| Acidity | |
| Low | 39 |
| Low-medium | 42 |
| Medium | 20 |
| Medium-high | 9 |
| High | 2 |
| Bitterness | |
| Low | 35 |
| Low-medium | 38 |
| Medium | 29 |
| Medium-high | 8 |
| High | 2 |
| Mouthfeel body | |
| Light | 9 |
| Light-medium | 34 |
| Medium | 53 |
| Medium-full | 12 |
| Full | 4 |
| Aftertaste intensity | |
| Short | 0 |
| Short-medium | 6 |
| Medium | 30 |
| Medium-long | 50 |
| Long | 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postigo, V.; García, M.; Cabellos, J.M.; Arroyo, T. Wine Saccharomyces Yeasts for Beer Fermentation. Fermentation 2021, 7, 290. https://doi.org/10.3390/fermentation7040290
Postigo V, García M, Cabellos JM, Arroyo T. Wine Saccharomyces Yeasts for Beer Fermentation. Fermentation. 2021; 7(4):290. https://doi.org/10.3390/fermentation7040290
Chicago/Turabian StylePostigo, Vanesa, Margarita García, Juan Mariano Cabellos, and Teresa Arroyo. 2021. "Wine Saccharomyces Yeasts for Beer Fermentation" Fermentation 7, no. 4: 290. https://doi.org/10.3390/fermentation7040290
APA StylePostigo, V., García, M., Cabellos, J. M., & Arroyo, T. (2021). Wine Saccharomyces Yeasts for Beer Fermentation. Fermentation, 7(4), 290. https://doi.org/10.3390/fermentation7040290