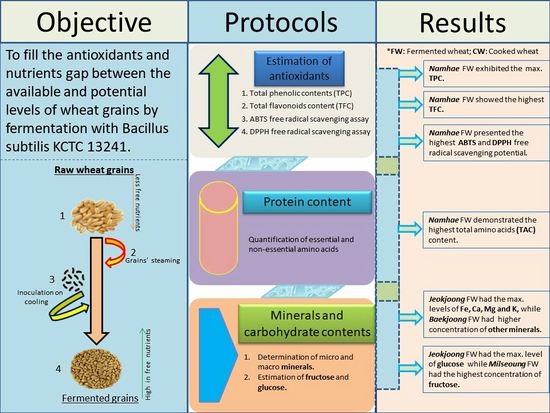
Relative Assessment of Biochemical Constituents and Antioxidant Potential of Fermented Wheat Grains Using Bacillus subtilis KCTC 13241
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Inoculum Preparation
2.3. Preparation of Fermented Wheat
2.4. Viable Cell Number
2.5. Sample Extraction
2.6. Determination of Total Phenolic Contents
2.7. Evaluation of Total Flavonoid Content
2.8. DPPH (2,2-diphenyl-1-picrylhydrazyl) Radical-Scavenging Assay
2.9. ABTS Cation Radical-Scavenging Assay
2.10. Free Amino Acids Composition
2.11. Analysis of Minerals and Carbohydrates
2.12. Statistical Analysis
3. Results and Discussion
3.1. Variation in Viable Microbial Population in Fermented and Cooked Wheat
3.2. Total Phenolic Contents (TPC) of Fermented and Cooked Wheat
3.3. Total Flavonoid Contents (TFC) of Fermented and Cooked Wheat
3.4. DPPH Free Radical Scavenging Activity
3.5. ABTS Radical Scavenging Potential of FW and CW Extract
3.6. Amino Acid Profile of FW and CW
3.7. Minerals and Carbohydrate Contents of FW and CW
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, F.; Chen, Z.; Shao, J.; Wang, C.; Zhan, C. Effect of fermentation on the peptide content, phenolics and antioxidant activity of defatted wheat germ. Food Biosci. 2017, 20, 141–148. [Google Scholar] [CrossRef]
- Dordević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Calliste, C.; Allais, D.; Simon, A.; Marfak, A.; Delage, C.; Duroux, J. Antioxidant, anti-inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas. Food Chem. 2003, 80, 399–407. [Google Scholar] [CrossRef]
- Cai, S.; Wang, O.; Wu, W.; Zhu, S.; Zhou, F.; Ji, B.; Gao, F.; Zhang, D. Comparative study of the effects of solid-state fermentation with three filamentous fungi on the total phenolics content (TPC), flavonoids, and antioxidant activities of subfractions from oats (Avena sativa L.). J. Agric. Food. Chem. 2012, 60, 507–513. [Google Scholar] [CrossRef]
- Juan, M.Y.; Chou, C.C. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiol. 2010, 27, 586–591. [Google Scholar] [CrossRef]
- Bhanja, T.; Kumari, A.; Banerjee, R. Enrichment of phenolics and free radical scavenging property of wheat koji prepared with two filamentous fungi. Bioresour. Technol. 2009, 100, 2861–2866. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.W.; Kim, I.D.; Bilal, S.; Shahzad, R.; Saeed, M.T.; Adhikari, B.; Nabi, R.B.S.; Kyo, J.R.; Shin, D.H. Effects of bacterial fermentation on the biochemical constituents and antioxidant potential of fermented and unfermented soybeans using probiotic bacillus subtilis (KCTC 13241). Molecules 2017, 22, 2200. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.W.; Shahzad, R.; Bilal, S.; Adhikari, B.; Kim, I.D.; Lee, J.D.; Lee, I.J.; Kim, B.O.; Shin, D.H. Comparison of antioxidants potential, metabolites, and nutritional profiles of Korean fermented soybean (Cheonggukjang) with Bacillus subtilis KCTC 13241. J. Food Sci. Technol. 2018, 55, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.W.; Ilays, M.Z.; Saeed, M.T.; Shin, D.H. Comparative assessment regarding antioxidative and nutrition potential of Moringa oleifera leaves by bacterial fermentation. J. Food Sci. Technol. 2020, 57, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef] [PubMed]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K.; Yamane, Y. Screening for Antioxidant Activity in Edible Plant Products: Comparison of Low-Density Lipoprotein Oxidation Assay, DPPH Radical Scavenging Assay, and Folin-Ciocalteu Assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Dhungana, S.K.; Waqas Ali, M.; Adhikari, A.; Kim, I.D.; Shin, D.H. Antioxidant activities, polyphenol, flavonoid, and amino acid contents in peanut shell. J. Saudi Soc. Agric. Sci. 2018, 18, 2–7. [Google Scholar] [CrossRef]
- Bilal, S.; Khan, A.L.; Waqas, M.; Shahzad, R.; Kim, I.D.; Lee, I.J.; Shin, D.H. Biochemical constituents and in vitro antioxidant and anticholinesterase potential of seeds from Native Korean Persimmon Genotypes. Molesafcscules 2016, 21, 893. [Google Scholar] [CrossRef] [Green Version]
- Andualem, B.; Gessesse, A. Proximate composition, mineral content and antinutritional factors of Brebra (Millettia ferruginea) seed flour as well as physicochemical characterization of its seed oil. Springerplus 2014, 3, 298. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.M.; Radhakrishnan, R.; You, Y.H.; Joo, G.J.; Lee, I.J.; Lee, K.E.; Kim, J.H. Phosphate Solubilizing Bacillus megaterium mj1212 Regulates Endogenous Plant Carbohydrates and Amino Acids Contents to Promote Mustard Plant Growth. Indian, J. Microbiol. 2014, 54, 427–433. [Google Scholar] [CrossRef]
- Hwang, I.S.; Kim, J.E.; Lee, Y.J.; Kwak, M.H.; Lee, H.G.; Kim, H.S.; Lee, H.S.; Hwang, D.Y. Growth sensitivity in the epiphyseal growth plate, liver and muscle of SD rats is significantly enhanced by treatment with a fermented soybean product (cheonggukjang) through stimulation of growth hormone secretion. Mol. Med. Rep. 2014, 9, 166–172. [Google Scholar] [CrossRef]
- Cho, K.M.; Lee, J.H.; Yun, H.D.; Ahn, B.Y.; Kim, H.; Seo, W.T. Changes of phytochemical constituents (isoflavones, flavanols, and phenolic acids) during cheonggukjang soybeans fermentation using potential probiotics Bacillus subtilis CS90. J. Food Compos. Anal. 2011, 24, 402–410. [Google Scholar] [CrossRef]
- Kun, S.; Rezessy-Szabó, J.M.; Nguyen, Q.D.; Hoschke, Á. Changes of microbial population and some components in carrot juice during fermentation with selected Bifidobacterium strains. Process Biochem. 2008, 43, 816–821. [Google Scholar] [CrossRef]
- Lee, D.; Kulkarni, K.P.; Kim, B.; Mi, Y.; Tae, J.; Lee, J. Comparative assessment of quality characteristics of Chungkookjang made from soybean seeds differing in oleic acid concentration. J. Funct. Foods 2019, 52, 529–536. [Google Scholar] [CrossRef]
- Katina, K.; Liukkonen, K.H.; Kaukovirta-Norja, A.; Adlercreutz, H.; Heinonen, S.M.; Lampi, A.M.; Pihlava, J.M.; Poutanen, K. Fermentation-induced changes in the nutritional value of native or germinated rye. J. Cereal Sci. 2007, 46, 348–355. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Chen, J.; Tang, H.; Wang, C.; Li, Z.; Xiao, Y. Bioprocessing of soybeans (Glycine maxL.) by solid-state fermentation withEurotium cristatumYL-1 improves total phenolic content, isoflavone aglycones, and antioxidant activity. RSC Adv. 2020, 10, 16928–16941. [Google Scholar] [CrossRef]
- Kariluoto, S.; Aittamaa, M.; Korhola, M.; Salovaara, H.; Vahteristo, L.; Piironen, V. Effects of yeasts and bacteria on the levels of folates in rye sourdoughs. Int. J. Food Microbiol. 2006, 106, 137–143. [Google Scholar] [CrossRef]
- Lee, I.H.; Hung, Y.H.; Chou, C.C. Solid-state fermentation with fungi to enhance the antioxidative activity, total phenolic and anthocyanin contents of black bean. Int. J. Food Microbiol. 2008, 121, 150–156. [Google Scholar] [CrossRef]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Yu, L.; Perret, J.; Davy, B.; Wilson, J.; Melby, C.L. Antioxidant properties of cereal products. J. Food Sci. 2002, 67, 2600–2603. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Kim, N.Y.; Song, E.J.; Kwon, D.Y.; Kim, H.P.; Heo, M.Y. Antioxidant and antigenotoxic activities of Korean fermented soybean. Food Chem. Toxicol. 2008, 46, 1184–1189. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Bhaskar, N. In vitro antioxidant activity of liquor from fermented shrimp biowaste. Bioresour. Technol. 2008, 99, 9013–9016. [Google Scholar] [CrossRef] [PubMed]
- Starzyńska-Janiszewska, A.; Stodolak, B.; Jamróz, M. Antioxidant properties of extracts from fermented and cooked seeds of Polish cultivars of Lathyrus sativus. Food Chem. 2008, 109, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.B.; Kuhad, R.C. Enhanced production and extraction of phenolic compounds from wheat by solid-state fermentation with Rhizopus oryzae RCK2012. Biotechnol. Rep. 2014, 4, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Niki, E.; Noguchi, N. Evaluation of antioxidant capacity. What capacity is being measured by which method? IUBMB Life 2000, 50, 323–329. [Google Scholar] [CrossRef]
- Hwang, C.E.; Seo, W.T.; Cho, K.M. Enhanced antioxidant effect of black soybean by Cheonggukjang with potential probiotic Bacillus subtilis CSY191. Korean J. Microbiol. 2013, 49, 391–397. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein Hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148–3161. [Google Scholar] [CrossRef] [Green Version]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Large neutral amino acids: Dietary effects on brain neurochemistry and function. Amino Acids 2013, 45, 419–430. [Google Scholar] [CrossRef]
- Lukaski, H.C. Vitamin and mineral status: Effects on physical performance. Nutrition 2004, 20, 632–644. [Google Scholar] [CrossRef]
- Maria John, K.M.; Enkhtaivan, G.; Lee, J.; Thiruvengadam, M.; Keum, Y.-S.; Kim, D.H. Spectroscopic determination of metabolic and mineral changes of soya-chunk mediated by Aspergillus sojae. Food Chem. 2015, 170, 1–9. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Prückler, M.; Lorenz, C.; Endo, A.; Kraler, M.; Dürrschmid, K.; Hendriks, K.; Soares da Silva, F.; Auterith, E.; Kneifel, W.; Michlmayr, H. Comparison of homo- and heterofermentative lactic acid bacteria for implementation of fermented wheat bran in bread. Food Microbiol. 2015, 49, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Codinǎ, G.G.; Mironeasa, S.; Voica, D.V.; Mironeasa, C. Multivariate analysis of wheat flour dough sugars, gas production, and dough development at different fermentation times. Czech J. Food Sci. 2013, 31, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Sahlström, S.; Park, W.; Shelton, D.R. Factors Influencing Yeast Fermentation and the Effect of LMW Sugars and Yeast Fermentation on Hearth Bread Quality. Cereal Chem. 2004, 81, 328–335. [Google Scholar] [CrossRef]





| Amino Acids mg/g | Sample | Baekjoong | Jeokjoong | Milseoung | Namhae |
|---|---|---|---|---|---|
| arginine | CW | 4.63 ± 0.98 | 4.93 ± 1.07 | 5.35 ± 1.34 | 5.43 ± 1.49 |
| FW | 5.14 ± 1.43 | 5.69 ± 1.64 | 6.43 ± 1.13 | 4.76 ± 0.45 | |
| threonine | CW | 2.42 ± 0.42 | 2.31 ± 0.36 | 2.65 ± 0.33 | 1.82 ± 0.37 |
| FW | 3.23 ± 0.67 | 3.73 ± 0.51 | 3.52 ± 1.02 | 2.39 ± 0.62 | |
| Serine | CW | 3.35 ± 0.30 | 4.13 ± 0.86 | 3.93 ± 0.24 | 4.05 ± 0.56 |
| FW | 4.24 ± 0.85 | 5.03 ± 1.12 | 4.37 ± 0.53 | 5.35 ± 1.68 | |
| glutamic acid | CW | 37.98 ± 3.86 | 35.29 ± 2.43 | 38.74 ± 4.63 | 38.99 ± 5.43 |
| FW | 45.65 ± 5.39 | 42.32 ± 3.78 | 48.80 ± 5.24 | 47.94 ± 4.94 | |
| phenylalanine | CW | 5.15 ± 1.87 | 5.37 ± 1.58 | 5.99 ± 0.71 | 4.65 ± 0.92 |
| FW | 6.76 ± 1.74 | 6.97 ± 1.49 | 6.54 ± 1.19 | 5.16 ± 1.57 | |
| Glycine | CW | 3.04 ± 0.68 | 3.45 ± 0.43 | 4.56 ± 0.92 | 4.81 ± 1.03 |
| FW | 4.72 ± 0.65 | 4.78 ± 1.04 | 5.41 ± 1.23 | 5.49 ± 1.31 | |
| Alanine | CW | 2.81 ± 0.71 | 2.23 ± 0.50 | 3.89 ± 0.69 | 4.82 ± 1.04 |
| FW | 3.46 ± 1.13 | 3.93 ± 1.23 | 4.25 ± 1.02 | 5.63 ± 1.78 | |
| histidine | CW | 2.96 ± 0.57 | 3.52 ± 1.07 | 4.53 ± 0.67 | 4.21 ± 1.18 |
| FW | 3.13 ± 0.59 | 4.13 ± 1.16 | 5.37 ± 0..48 | 5.04 ± 1.24 | |
| Valine | CW | 3.59 ± 0.12 | 4.58 ± 1.21 | 5.01 ± 144 | 4.17 ± 0.72 |
| FW | 3.99 ± 0.64 | 5.16 ± 1.67 | 6.05 ± 0.89 | 5.99 ± 1.13 | |
| methionine | CW | 1.48 ± 0.54 | 1.87 ± 0.67 | 1.95 ± 0.59 | 2.54 ± 0.81 |
| FW | 2.46 ± 0.52 | 2.47 ± 0.83 | 2.89 ± 0.83 | 3.69 ± 1.48 | |
| aspartic acid | CW | 4.67± 0.21 | 3.62 ± 0.16 | 4.98 ± 0.24 | 5.16 ± 0.29 |
| FW | 5.25 ± 1.54 | 3.98 ± 1.23 | 5.23 ± 1.38 | 5.82 ± 1.67 | |
| isoleucine | CW | 2.98 ± 1.23 | 3.26 ± 0.76 | 3.72 ± 0.89 | 4.14 ± 0.51 |
| FW | 3.87 ± 1.71 | 4.78 ± 0.62 | 4.57 ± 0.82 | 5.68 ± 1.37 | |
| Leucine | CW | 6.45 ± 1.13 | 4.33 ± 0.31 | 6.79 ± 1.37 | 7.15 ± 0.92 |
| FW | 7.39 ± 1.05 | 5.31 ± 1.87 | 7.75 ± 0.95 | 8.31 ± 1.82 | |
| Tyrosine | CW | 2.51 ± 0.76 | 2.86 ± 0.63 | 3.24 ± 0.43 | 3.98 ± 0.74 |
| FW | 3.49 ± 1.15 | 3.65 ± 0.84 | 4.75 ± 1.08 | 5.46 ± 1.39 | |
| Total Amino Acids | CW | 84.02 ± 4.87 | 81.48 ± 5.32 | 95.33 ± 6.76 | 95.94 ± 5.98 |
| FW | 102.78 ± 5.32 | 101.93 ± 4.13 | 115.91 ± 5.17 | 116.71 ± 4.64 |
| Minerals mg/100 g | Sample | Baekjoong | Jeokjoong | Milseoung | Namhae |
|---|---|---|---|---|---|
| Iron | CW | 3.17 ± 0.23 | 3.26 ± 0.13 | 3.25 ± 0.14 | 3.11 ± 0.22 |
| FW | 3.98 ± 0.89 | 4.71 ± 0.32 | 4.73 ± 0.28 | 3.26 ± 0.85 | |
| Calcium | CW | 25.58 ± 3.83 | 28.43 ± 3.64 | 23.65 ± 4.49 | 23.89 ± 4.63 |
| FW | 29.87 ± 2.23 | 30.87 ± 2.77 | 27.35 ± 3.43 | 26.39 ± 3.98 | |
| Phosphorus | CW | 287.77 ± 7.32 | 285.43 ± 4.92 | 278.49 ± 4.82 | 275.15 ± 5.94 |
| FW | 315.32 ± 5.75 | 302.54 ± 5.98 | 295.45 ± 5.98 | 300.24 ± 6.47 | |
| Magnesium | CW | 120.87 ± 6.41 | 124.29 ± 7.73 | 123.99 ± 5.13 | 123.04 ± 7.15 |
| FW | 139.11 ± 5.34 | 145.87 ± 9.65 | 140.62 ± 7.60 | 131.82 ± 8.73 | |
| Selenium | CW | 71.04 ± 6.21 | 70.65 ± 3.76 | 71.41 ± 3.29 | 70.25 ± 6.37 |
| FW | 70.65 ± 4.19 | 69.78 ± 4.65 | 72.76 ± 7.13 | 68.92 ± 5.92 | |
| Potassium | CW | 356.22 ± 6.94 | 360.32 ± 9.54 | 351.73 ± 7.82 | 349.14 ± 7.81 |
| FW | 368.71 ± 7.15 | 385.12 ± 10.07 | 388.60 ± 10.54 | 365.84 ± 8.95 | |
| Manganese | CW | 3.99 ± 0.43 | 3.28 ± 1.09 | 2.91 ± 0.84 | 2.27 ± 0.32 |
| FW | 4.13 ± 0.74 | 4.96 ± 0.61 | 3.65 ± 0.73 | 3.99 ± 0.36 | |
| Zinc | CW | 1.48 ± 0.54 | 2.19 ± 0.67 | 1.95 ± 0.59 | 2.54 ± 0.81 |
| FW | 2.46 ± 0.52 | 2.47 ± 0.83 | 2.89 ± 0.83 | 3.69 ± 1.48 | |
| Sodium | CW | 2.32 ± 0.19 | 2.26 ± 0.87 | 2.25 ± 0.15 | 2.30 ± 0.95 |
| FW | 4.78 ± 0.84 | 4.00 ± 0.90 | 4.49 ± 0.36 | 4.02 ± 0.72 | |
| Copper | CW | 0.45 ± 0.17 | 0.33 ± 0.09 | 0.32 ± 0.01 | 0.39 ± 0.43 |
| FW | 0.73 ± 0.07 | 0.63 ± 0.05 | 0.41 ± 0.11 | 0.72 ± 0.17 | |
| Glucose/100 g | CW | 0.41 ± 0.06 | 0.46 ± 0.13 | 0.23 ± 0.09 | 0.19 ± 0.10 |
| FW | 0.42 ± 0.12 | 0.65 ± 0.17 | 0.25 ± 0.08 | 0.21 ± 0.04 | |
| Fructose/100 g | CW | 71.02 ± 3.27 | 65.38 ± 5.32 | 67.27 ± 4.63 | 64.12 ± 4.76 |
| FW | 72.24 ± 3.81 | 75.42 ± 6.87 | 90.54 ± 6.17 | 76.13 ± 5.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyas, M.Z.; Lee, J.K.; Ali, M.W.; Tariq, S.; Nadeem, M. Relative Assessment of Biochemical Constituents and Antioxidant Potential of Fermented Wheat Grains Using Bacillus subtilis KCTC 13241. Fermentation 2022, 8, 113. https://doi.org/10.3390/fermentation8030113
Ilyas MZ, Lee JK, Ali MW, Tariq S, Nadeem M. Relative Assessment of Biochemical Constituents and Antioxidant Potential of Fermented Wheat Grains Using Bacillus subtilis KCTC 13241. Fermentation. 2022; 8(3):113. https://doi.org/10.3390/fermentation8030113
Chicago/Turabian StyleIlyas, Muhammad Zahaib, Ju Kyong Lee, Muhammad Waqas Ali, Sana Tariq, and Muhammad Nadeem. 2022. "Relative Assessment of Biochemical Constituents and Antioxidant Potential of Fermented Wheat Grains Using Bacillus subtilis KCTC 13241" Fermentation 8, no. 3: 113. https://doi.org/10.3390/fermentation8030113
APA StyleIlyas, M. Z., Lee, J. K., Ali, M. W., Tariq, S., & Nadeem, M. (2022). Relative Assessment of Biochemical Constituents and Antioxidant Potential of Fermented Wheat Grains Using Bacillus subtilis KCTC 13241. Fermentation, 8(3), 113. https://doi.org/10.3390/fermentation8030113