Intensification of Acidogenic Fermentation for the Production of Biohydrogen and Volatile Fatty Acids—A Perspective
Abstract
:1. Introduction
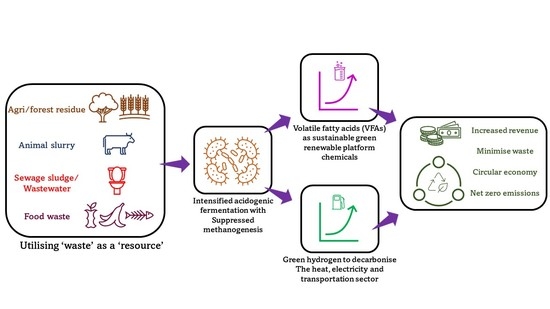
1.1. ‘Waste’ to ‘Value’ for Approaching a Circular Economy
1.2. Green Hydrogen and VFAs—Need for Process Intensification
- Pre-digestion—strategies to address feedstock heterogeneity and improve the bioavailability of the biomass;
- Anaerobic digestion—strategies to improve the bioconversion of biomass to desired products;
- Product recovery—strategies to maximise recovery and purity of desired products.
2. Pre-Digestion

2.1. Substrate Pre-Treatment to Enhance Biohydrogen Production
| Feedstock | Pre-Treatment Conditions | Digestion Conditions | Influence on H2 Yield | Reference |
|---|---|---|---|---|
| Physical Pre-treatment | ||||
| Chopped dried rice straw | 20 g/L straw, 2 L, high-speed disperser at 12,000 rpm, 30 min, 0.3 mm gap between rotor and stator | 1 L batch, 25% inoculum, 70% straw slurry, 37 °C, 100 rpm, 10 days | 7.3-fold increase in specific H2 yield | [49] |
| Chemical Pre-treatment | ||||
| Air-dried and milled corn cobs | 5 g biomass, 0.1 L pH 11.5 NaOH, 25 °C, 6 h, followed by enzymatic hydrolysis with Viscozyme L and glucosidase (0.001 L/g biomass), 42 °C, 24 h | 1 L batch, 10% v/v inoculum, pH 7, 37 °C, 320 rpm, 116 h | >5-fold increase in H2 yield | [52] |
| Grass silage | 2% silage, 0.1 L, 2% H2SO4, 135 °C, 15 min | 1% silage, 0.2 L batch, 0.02 L inoculum, pH 7, 4 days (1st stage of a 2-stage system) | 3-fold increase in H2 yield | [50] |
| Milled wheat straw | 5 g straw, 40% water, 0.75 bars, 0.63 LPM O3, 45 min | 0.08 L, 2 g TS, pH 6, 1.9% inoculum (v/v), 1 mL hydrolytic enzyme mix, 35 °C, 60 rpm, 8 days | ~2.5-fold increase in cumulative H2 yield | [53] |
| Milled wheat straw | 5 g VS, 62.5 mL 80 mM Ca(OH)2, 20 °C, 2 days | 0.5 L, 8% w/v inoculum, 1 mL Accelerase-1500, pH 6.25, 35 °C, 16 days | ~29-fold increase in specific H2 yield | [51] |
| Biological Pre-treatment | ||||
| Blended food waste | 0.5 L, bioelectrochemical hydrolysis, open to air graphite cathode, graphite anode, 0.075 L inoculum, 20 g COD/L, pH 7, 10 h HRT, 29 °C | 0.25 L fed-batch, 0.075 L inoculum, 10 g/L, pH 6, 72 h HRT, 29 °C | ~35% increase in cumulative H2 yield | [55] |
| Cassava wastewater | 0.2% OPTIMASH BG® enzyme, 0.22 L wastewater, pH 4, 60 °C, 45 rpm | 0.06 L, substrate to inoculum ratio 5 (v/v basis), pH 7, 37 °C, 90 rpm, 10 days | Reduced lag time, 51% increase in specific H2 yield | [54] |
| 0.2% α-amylase enzyme, 0.22 L wastewater, 37 °C, 45 rpm | Reduced lag time, 49% increase in specific H2 yield | |||
| Physico-chemical Pre-treatment | ||||
| Commercial Sake Lees | 10% biomass, 0.1 L, 130 °C, 3 bars, 1 h | 0.11 L batch, 9% biomass, substrate to inoculum ratio of 1:1 v/v, pH 6, 75 rpm, 37 °C, 5 days | Reduction in lag time observed after pre-treatment | [56] |
| Marine macroalgae Ulva reticulate | Acidic H2O2 induced microwave, 0.5 L, 2% biomass, 0.024 g H2O2/g TS, 0.1 N H2SO4, pH 5, 40% microwave power, 10 min, 10.8 MJ/kg TS | 0.15 L batch, 70% substrate, 25% inoculum, pH 5.5, 130 rpm, 37 °C, | 7.7-fold increase in specific H2 yield | [59] |
| Waste-activated sludge | 0.15 L sludge, 0.3 g sodium citrate/g sludge, 1 h, 150 rpm, followed by 121 °C, 30 min | 0.2 L batch, substrate to inoculum ratio 3 (v/v basis), pH 7, 100 rpm, 37 °C | 4.4-fold increase in specific H2 yield | [58] |
| Antibiotic fermentation residue | 0.2 L, 6 mm sonication probe, 30 min, 4 s ON 6 s OFF, followed by 5 M NaOH addition to reach pH 10, mixed for 24 h | 0.2 L batch, substrate to inoculum ratio 3 (v/v basis), pH 7, 37 °C | 79% increase in specific H2 yield | [62] |
2.2. Substrate Pre-Treatment to Enhance VFA Production
| Feedstock | Pre-Treatment Conditions | Digestion Conditions | Influence on VFA Yield | Reference |
|---|---|---|---|---|
| Physical Pre-treatment | ||||
| Waste-activated sludge | 0.45 L, 5 cycles of freezing and thawing, −24 °C freezing for 8 h, 35 °C thawing for 2 h | 1 L fed-batch, sludge-to-inoculum ratio of 2 (w/w), 80 rpm, 25 days retention time, 35 °C | 35% increase in maximum VFA concentration | [64] |
| Waste-activated sludge | 0.5 L, graphite electrodes, 15 V, pH 6.7, 30 min, 25 °C | 0.06 L sludge, 0.02 L inoculum, 35 °C, 60 rpm, 35 days | Suppressed CH4 production, ~100-fold increase in specific VFA yield | [65] |
| Chemical Pre-treatment | ||||
| Waste-activated sludge | 0.8 L feedstock, pH 10 (2 M NaOH), 0.5 g/g VSS K2FeO4, 120 rpm, 60 min | 0.4 L batch, 10% v/v inoculum, 160 rpm, 35 °C, 12 days | ~2.4-fold increase in maximum VFA concentration | [67] |
| Air-dried and chopped macroalgae | 40% TS, 0.5 N NaOH, 18 h | 0.1 L batch, 4% TS feedstock, 10% inoculum, 35 °C, 150 rpm, 4 days | 2-fold increase in maximum VFA concentration | [66] |
| Grass waste | 0.2 L, 5% grass, 1.75% carbide slag, 120 °C, 40 min | 0.25 L batch, substrate-to-inoculum ratio 2 (VS basis), pH 7, 100 rpm, 35 °C, 14 days | 0.6–2.4-fold increase in maximum VFA concentration | [68] |
| Sludge | 0.5 L sludge, 20 mg/g tetrakis hydroxymethyl phosphonium sulphate, 2 days, 150 rpm, 30 °C | 0.35 L sludge batch, 0.03 L inoculum, pH 6, 2 days, 150 rpm, 30 °C | 4-fold increase in maximum VFA concentration | [69] |
| Biological Pre-treatment | ||||
| Autoclaved solid digestate | 100 g TS, 10 g white rot fungi Pleurotus Sajor-Caju, 25 °C, 70% relative humidity, 6 weeks | 0.4 L batch, 15% TS, inoculum-to-substrate ratio 2 (TS basis), 30 °C, 18 days | 1.2-fold increase in maximum VFA concentration | [71] |
| Air-dried and chopped macroalgae | 4% TS, 0.09 L, Vibrio spp., 26–30 °C, 2 days | 0.1 L batch, 4% TS feedstock, 10% inoculum, 35 °C, 150 rpm, 4 days | 2.5-fold increase in maximum VFA concentration | [66] |
| Primary sludge | 1% Novozym 50199 to biomass, 300 rpm, 10 min | 0.5 L fed-batch, 2-day retention time, 25 °C | 56% increase in maximum VFA concentration | [73] |
| Physico-chemical Pre-treatment | ||||
| Crushed food waste | 0.3 L feedstock, 8 mm 20 kHz sonication probe, 1 W/mL, 20 min | 0.18 L batch, substrate to inoculum ratio 6 (VS basis), 180 rpm, 35 °C, 5 days | ~4.3-fold increase in maximum VFA concentration | [78] |
| Lipid-extracted microalgae Ettlia sp. | 10 mL of 5% microalgal slurry, 1% NaOH, 25% amplitude sonication | 0.1 L batch, 3% TS, 20% v/v inoculum, pH 7.2, 150 rpm, 35 °C, 7 days | 30% increase in maximum VFA concentration | [76] |
| 10 mL of 5% microalgal slurry, 1% NaOH, microwave | 10% increase in maximum VFA concentration | |||
| 10 mL of 5% microalgal slurry, 1% NaOH, autoclave 121 °C, 1 h, 1 bar | 20% increase in maximum VFA concentration | |||
| Thickened waste-activated sludge | 1 L sludge, 190 °C, 10 min, 12.5 bars | 0.3 L batch, 1 gTCOD/gVSS substrate to inoculum ratio, pH 5.5, 120 rpm, 37 °C, 3 days | 3-fold increase in maximum VFA concentration | [75] |
| Waste-activated sludge | 0.2 L sludge, 0.01 g sodium dodecylbenzene sulfonate/g TS, 70 °C, 1 h, 400 rpm | 0.2 L batch, 150 rpm, 37 °C, 7 days | 4-fold increase in maximum VFA concentration | [81] |
| Grass clippings | 0.1 L, 2% grass, 0.75% Ca(OH)2, sonication at 2.5 W/mL for 10 min (5 s ON 5 s OFF pulse) | Solids and liquids were separated and fermented, 0.2 L batch, pH 7, 120 rpm, 35 °C, 12 days | ~2.1-fold increase in maximum VFA concentration | [80] |
| Waste-activated sludge and vegetable/fruit waste | Rotor-stator hydrodynamic cavitation, 2 bars inlet pressure, 80–100 L/min inflow rate, 1450–1550 rpm rotor speed, 50 min | 4 L batch, substrate-to-inoculum ratio 6–7 (VS basis), 37 °C, 14 rpm | ~9-fold increase in maximum VFA concentration | [46] |
3. Anaerobic Digestion for the Production of Biohydrogen or VFAs
3.1. Hydraulic Retention Time (HRT)
3.2. Organic Loading Rate (OLR)
3.3. pH
3.4. Temperature
3.5. Operational Mode and Reactor Configuration
3.5.1. CSTR
3.5.2. AFBR
3.5.3. ASBR
3.5.4. APBR
3.5.5. UASBR
3.6. Additives
3.7. Undesired By-Products and Inhibitors
4. Product Recovery
5. Techno-Economics and Process Life Cycle
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. Data and Statistics. Available online: https://www.iea.org/data-and-statistics/data-browser?country=UK&fuel=CO2%20emissions&indicator=CO2BySector (accessed on 3 July 2022).
- Nagarajan, S.; Ranade, V.V. Valorizing Waste Biomass via Hydrodynamic Cavitation and Anaerobic Digestion. Ind. Eng. Chem. Res. 2021, 60, 16577–16598. [Google Scholar] [CrossRef]
- Demir, M.E.; Chehade, G.; Dincer, I.; Yuzer, B.; Selcuk, H. Design and analysis of a new system for photoelectrochemical hydrogen production from wastewater. Energy Convers. Manag. 2019, 199, 111903. [Google Scholar] [CrossRef]
- Nagarajan, S.; Skillen, N.; Robertson, P.; Lawton, L. Cellulose Photocatalysis for Renewable Energy Production. In Metal, Metal-Oxides and Metal Sulfides for Batteries, Fuel Cells, Solar Cells, Photocatalysis and Health Sensors; Rajendran, S., Karimi-Maleh, H., Qin, J., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–34. [Google Scholar]
- Nagarajan, S.; Skillen, N.C.; Irvine, J.T.S.; Lawton, L.A.; Robertson, P.K.J. Cellulose II as bioethanol feedstock and its advantages over native cellulose. Renew. Sustain. Energy Rev. 2017, 77, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Skillen, N.; Nagarajan, S.; Ralphs, K.; Irvine, J.T.S.; Lawton, L.; Robertson, P.K.J. Using cellulose polymorphs for enhanced hydrogen production from photocatalytic reforming. Sustain. Energy Fuels 2019, 3, 1971–1975. [Google Scholar] [CrossRef]
- Wakerley, D.W.; Kuehnel, M.F.; Orchard, K.L.; Ly, K.H.; Rosser, T.E.; Reisner, E. Solar-driven reforming of lignocellulose to H2 with a CdS/CdOx photocatalyst. Nat. Energy 2017, 2, 17021. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, S. Development of Photocatalytic Reactor Technology for the Production of Fermentable Sugars. Ph.D. Thesis, Queen’s University, Belfast, Ireland, 2017. [Google Scholar]
- Meynell, P.J. Methane: Planning a Digester; Schocken Books: New York, NY, USA, 1976. [Google Scholar]
- NNFCC. Biomethane RTFC Calculator. Available online: https://www.nnfcc.co.uk/publications/tool-biomethane-rtfc-calculator (accessed on 6 April 2022).
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient Recovery from Digestate: Systematic Technology Review and Product Classification. Waste Biomass Valorization 2017, 8, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Kang, K.; Klinghoffer, N.B.; ElGhamrawy, I.; Berruti, F. Thermochemical conversion of agroforestry biomass and solid waste using decentralized and mobile systems for renewable energy and products. Renew. Sustain. Energy Rev. 2021, 149, 111372. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.-H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic thermochemical conversion of biomass for biofuel production: A comprehensive review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Nagarajan, S.; Stella, L.; Lawton, L.A.; Irvine, J.T.S.; Robertson, P.K.J. Mixing regime simulation and cellulose particle tracing in a stacked frame photocatalytic reactor. Chem. Eng. J. 2017, 313, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-W.; Nguyen, B.-S.; Wu, J.C.S.; Nguyen, V.-H. A current perspective for photocatalysis towards the hydrogen production from biomass-derived organic substances and water. Int. J. Hydrogen Energy 2020, 45, 18144–18159. [Google Scholar] [CrossRef]
- Lu, X.; Xie, S.; Yang, H.; Tong, Y.; Ji, H. Photoelectrochemical hydrogen production from biomass derivatives and water. Chem. Soc. Rev. 2014, 43, 7581–7593. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Kamarudin, S.K.; Minggu, L.J. Biofuel from biomass via photo-electrochemical reactions: An overview. J. Power Sources 2014, 259, 33–42. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current perspectives on acidogenic fermentation to produce volatile fatty acids from waste. Rev. Environ. Sci. Bio/Technol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- UK BEIS. The Ten Point Plan for a Green Industrial Revolution. 2020; Volume 38. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/936567/10_POINT_PLAN_BOOKLET.pdf (accessed on 7 April 2022).
- UK BEIS. UK Hydrogen Strategy. 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1011283/UK-Hydrogen-Strategy_web.pdf (accessed on 7 April 2022).
- UK BEIS. Industrial Decarbonisation Strategy. 2021. Available online: https://www.gov.uk/government/publications/industrial-decarbonisation-strategy (accessed on 7 April 2022).
- UK DEFRA. A Green Future: Our 25 Year Plan to Improve the Environment. 2018. Available online: https://www.gov.uk/government/publications/25-year-environment-plan (accessed on 7 April 2022).
- European Commission. A Hydrogen Strategy for a Climate-Neutral Europe. 2020. Available online: https://ec.europa.eu/energy/sites/ener/files/hydrogen_strategy.pdf (accessed on 8 April 2022).
- Woodward, E.; Han, O.; Forbes, C. Enabling the European Hydrogen Economy; Aurora Energy Research: Oxford, UK, 2021. [Google Scholar]
- International Energy Agency. Global Hydrogen Review; IEA: Paris, France, 2021; p. 224. [Google Scholar]
- Braga, L.B.; Silveira, J.L.; da Silva, M.E.; Tuna, C.E.; Machin, E.B.; Pedroso, D.T. Hydrogen production by biogas steam reforming: A technical, economic and ecological analysis. Renew. Sustain. Energy Rev. 2013, 28, 166–173. [Google Scholar] [CrossRef]
- Gorski, J.; Jutt, T.; Tam Wu, K. Carbon intensity of blue hydrogen production. Pembin. Inst. 2021. Available online: https://www.pembina.org/reports/carbon-intensity-of-blue-hydrogen-revised.pdf (accessed on 16 June 2022).
- Ali Khan, M.H.; Daiyan, R.; Neal, P.; Haque, N.; MacGill, I.; Amal, R. A framework for assessing economics of blue hydrogen production from steam methane reforming using carbon capture storage & utilisation. Int. J. Hydrogen Energy 2021, 46, 22685–22706. [Google Scholar]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas fermentation process development for production of biofuels and chemicals: A review. Bioresour. Technol. Rep. 2019, 7, 100279. [Google Scholar]
- Ewing, M.; Israel, B.; Jutt, T.; Talebian, H.; Stepanik, L. Hydrogen on the Path to Net-Zero Emissions–Costs and Climate Benefits; Pembina Institute: Toronto, ON, Canada, 2020; p. 6. [Google Scholar]
- Hitam, C.N.C.; Jalil, A.A. A review on biohydrogen production through photo-fermentation of lignocellulosic biomass. Biomass Convers. Biorefin. 2020, 1–19. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar]
- Bundhoo, Z.M.A. Potential of bio-hydrogen production from dark fermentation of crop residues: A review. Int. J. Hydrogen Energy 2019, 44, 17346–17362. [Google Scholar]
- Rajesh Banu, J.; Merrylin, J.; Mohamed Usman, T.M.; Yukesh Kannah, R.; Gunasekaran, M.; Kim, S.-H.; Kumar, G. Impact of pretreatment on food waste for biohydrogen production: A review. Int. J. Hydrogen Energy 2020, 45, 18211–18225. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Loh, K.-C. Review and perspectives of enhanced volatile fatty acids production from acidogenic fermentation of lignocellulosic biomass wastes. Bioresour. Bioprocess. 2021, 8, 68. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Q.; Zhang, Z.; Jing, Y.; Hu, J.; He, C.; Lu, C. A review on biological recycling in agricultural waste-based biohydrogen production: Recent developments. Bioresour. Technol. 2022, 347, 126595. [Google Scholar] [PubMed]
- Miao, Z.; Grift, T.E.; Hansen, A.C.; Ting, K.C. Energy requirement for comminution of biomass in relation to particle physical properties. Ind. Crops Prod. 2011, 33, 504–513. [Google Scholar] [CrossRef]
- Konde, K.S.; Nagarajan, S.; Kumar, V.; Patil, S.V.; Ranade, V.V. Sugarcane bagasse based biorefineries in India: Potential and challenges. Sustain. Energy Fuels 2021, 5, 52–78. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Bundhoo, M.A.Z.; Mohee, R.; Hassan, M.A. Effects of pre-treatment technologies on dark fermentative biohydrogen production: A review. J. Environ. Manag. 2015, 157, 20–48. [Google Scholar] [CrossRef]
- Cheng, X.-Y.; Liu, C.-Z. Fungal pretreatment enhances hydrogen production via thermophilic fermentation of cornstalk. Appl. Energy 2012, 91, 1–6. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Garuti, M.; Langone, M.; Fabbri, C.; Piccinini, S. Monitoring of full-scale hydrodynamic cavitation pretreatment in agricultural biogas plant. Bioresour. Technol. 2018, 247, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Lanfranchi, A.; Tassinato, G.; Valentino, F.; Martinez, G.A.; Jones, E.; Gioia, C.; Bertin, L.; Cavinato, C. Hydrodynamic cavitation pre-treatment of urban waste: Integration with acidogenic fermentation, PHAs synthesis and anaerobic digestion processes. Chemosphere 2022, 301, 134624. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Ranade, V.V. Pre-treatment of distillery spent wash (vinasse) with vortex based cavitation and its influence on biogas generation. Bioresour. Technol. Rep. 2020, 11, 100480. [Google Scholar] [CrossRef]
- Nagarajan, S.; Ranade, V.V. Pretreatment of Lignocellulosic Biomass Using Vortex-Based Devices for Cavitation: Influence on Biomethane Potential. Ind. Eng. Chem. Res. 2019, 58, 15975–15988. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Kavitha, S.; Sivashanmugham, P.; Kumar, G.; Nguyen, D.D.; Chang, S.W.; Rajesh Banu, J. Biohydrogen production from rice straw: Effect of combinative pretreatment, modelling assessment and energy balance consideration. Int. J. Hydrogen Energy 2019, 44, 2203–2215. [Google Scholar] [CrossRef]
- Deng, C.; Lin, R.; Cheng, J.; Murphy, J.D. Can acid pre-treatment enhance biohydrogen and biomethane production from grass silage in single-stage and two-stage fermentation processes? Energy Convers. Manag. 2019, 195, 738–747. [Google Scholar] [CrossRef]
- Reilly, M.; Dinsdale, R.; Guwy, A. Mesophilic biohydrogen production from calcium hydroxide treated wheat straw. Int. J. Hydrogen Energy 2014, 39, 16891–16901. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Konopacka-Łyskawa, D.; Słupek, E.; Makoś, P.; Cieśliński, H.; Kamiński, M. Influence of alkaline and oxidative pre-treatment of waste corn cobs on biohydrogen generation efficiency via dark fermentation. Biomass Bioenergy 2020, 141, 105691. [Google Scholar] [CrossRef]
- Wu, J.; Upreti, S.; Ein-Mozaffari, F. Ozone pretreatment of wheat straw for enhanced biohydrogen production. Int. J. Hydrogen Energy 2013, 38, 10270–10276. [Google Scholar] [CrossRef]
- Leaño, E.P.; Babel, S. Effects of pretreatment methods on cassava wastewater for biohydrogen production optimization. Renew. Energy 2012, 39, 339–346. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Venkata Mohan, S. Bio-electrohydrolysis as a pretreatment strategy to catabolize complex food waste in closed circuitry: Function of electron flux to enhance acidogenic biohydrogen production. Int. J. Hydrogen Energy 2014, 39, 11411–11422. [Google Scholar] [CrossRef]
- Choiron, M.; Tojo, S.; Chosa, T. Biohydrogen production improvement using hot compressed water pretreatment on sake brewery waste. Int. J. Hydrogen Energy 2020, 45, 17220–17232. [Google Scholar] [CrossRef]
- Asadi, N.; Zilouei, H. Optimization of organosolv pretreatment of rice straw for enhanced biohydrogen production using Enterobacter aerogenes. Bioresour. Technol. 2017, 227, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, J. Enhancing biohydrogen production from disintegrated sewage sludge by combined sodium citrate-thermal pretreatment. J. Cleaner Prod. 2021, 312, 127756. [Google Scholar] [CrossRef]
- Dinesh Kumar, M.; Kaliappan, S.; Gopikumar, S.; Zhen, G.; Rajesh Banu, J. Synergetic pretreatment of algal biomass through H2O2 induced microwave in acidic condition for biohydrogen production. Fuel 2019, 253, 833–839. [Google Scholar] [CrossRef]
- Salem, A.H.; Brunstermann, R.; Mietzel, T.; Widmann, R. Effect of pre-treatment and hydraulic retention time on biohydrogen production from organic wastes. Int. J. Hydrogen Energy 2018, 43, 4856–4865. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J. Pretreatment of macroalgal Laminaria japonica by combined microwave-acid method for biohydrogen production. Bioresour. Technol. 2018, 268, 52–59. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, Y.; Wang, J. Pretreatment of antibiotic fermentation residues by combined ultrasound and alkali for enhancing biohydrogen production. J. Clean. Prod. 2020, 268, 122190. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Ultrasonic pretreatment for an enhancement of biohydrogen production from complex food waste. Int. J. Hydrogen Energy 2014, 39, 7721–7729. [Google Scholar] [CrossRef]
- She, Y.; Hong, J.; Zhang, Q.; Chen, B.-Y.; Wei, W.; Xin, X. Revealing microbial mechanism associated with volatile fatty acids production in anaerobic acidogenesis of waste activated sludge enhanced by freezing/thawing pretreatment. Bioresour. Technol. 2020, 302, 122869. [Google Scholar] [CrossRef]
- Zeng, Q.; Zan, F.; Hao, T.; Khanal, S.K.; Chen, G. Sewage sludge digestion beyond biogas: Electrochemical pretreatment for biochemicals. Water Res. 2022, 208, 117839. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Um, Y.; Yoon, H.H. Pretreatment of macroalgae for volatile fatty acid production. Bioresour. Technol. 2013, 146, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, J.; Wang, M.; Xin, X.; Xu, J.; Zhang, J. Efficient Volatile Fatty Acids Production from Waste Activated Sludge after Ferrate Pretreatment with Alkaline Environment and the Responding Microbial Community Shift. ACS Sustain. Chem. Eng. 2018, 6, 16819–16827. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, P.; Zhang, G.; Nabi, M.; Wang, S.; Ye, J.; Bao, S.; Zhang, Q.; Chen, N. Carbide slag pretreatment enhances volatile fatty acid production in anaerobic fermentation of four grass biomasses. Energy Convers. Manag. 2019, 199, 112009. [Google Scholar] [CrossRef]
- Wu, Q.-L.; Guo, W.-Q.; Bao, X.; Yin, R.-L.; Feng, X.-C.; Zheng, H.-S.; Luo, H.-C.; Ren, N.-Q. Enhancing sludge biodegradability and volatile fatty acid production by tetrakis hydroxymethyl phosphonium sulfate pretreatment. Bioresour. Technol. 2017, 239, 518–522. [Google Scholar] [CrossRef]
- Han, F.; Wu, L. Comprehensive Utilization of Carbide Slag. In Industrial Solid Waste Recycling in Western China; Han, F., Wu, L.E., Eds.; Springer: Singapore, 2019; pp. 357–391. [Google Scholar]
- Fang, W.; Zhang, P.; Zhang, X.; Zhu, X.; van Lier, J.B.; Spanjers, H. White rot fungi pretreatment to advance volatile fatty acid production from solid-state fermentation of solid digestate: Efficiency and mechanisms. Energy 2018, 162, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Zhang, X.; Zhang, P.; Carol Morera, X.; van Lier, J.B.; Spanjers, H. Evaluation of white rot fungi pretreatment of mushroom residues for volatile fatty acid production by anaerobic fermentation: Feedstock applicability and fungal function. Bioresour. Technol. 2020, 297, 122447. [Google Scholar] [CrossRef]
- Bahreini, G.; Nazari, L.; Ho, D.; Flannery, C.C.; Elbeshbishy, E.; Santoro, D.; Nakhla, G. Enzymatic pre-treatment for enhancement of primary sludge fermentation. Bioresour. Technol. 2020, 305, 123071. [Google Scholar] [CrossRef]
- Owusu-Agyeman, I.; Balachandran, S.; Plaza, E.; Cetecioglu, Z. Co-fermentation of municipal waste streams: Effects of pretreatment methods on volatile fatty acids production. Biomass Bioenergy 2021, 145, 105950. [Google Scholar] [CrossRef]
- Kakar, F.l.; Koupaie, E.H.; Razavi, A.S.; Hafez, H.; Elbeshbishy, E. Effect of Hydrothermal Pretreatment on Volatile Fatty Acids Production from Thickened Waste Activated Sludge. BioEnergy Res. 2020, 13, 591–604. [Google Scholar] [CrossRef]
- Suresh, A.; Seo, C.; Chang, H.N.; Kim, Y.-C. Improved volatile fatty acid and biomethane production from lipid removed microalgal residue (LRμAR) through pretreatment. Bioresour. Technol. 2013, 149, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ma, H.; Boer, E.d.; Wu, W.; Wang, Q.; Gao, M.; Vo, D.-V.N.; Guo, M.; Xia, C. Effect of microwave/hydrothermal combined ionic liquid pretreatment on straw: Rumen anaerobic fermentation and enzyme hydrolysis. Environ. Res. 2022, 205, 112453. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wu, Q.; Yang, S.; Luo, H.; Peng, S.; Ren, N. Optimization of ultrasonic pretreatment and substrate/inoculum ratio to enhance hydrolysis and volatile fatty acid production from food waste. RSC Adv. 2014, 4, 53321–53326. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, J.; Yan, F.; Gao, Y.; Meng, Y.; Aihemaiti, A.; Ju, T. Enhancement of volatile fatty acid production and biogas yield from food waste following sonication pretreatment. J. Environ. Manag. 2018, 217, 797–804. [Google Scholar] [CrossRef]
- Wang, S.; Tao, X.; Zhang, G.; Zhang, P.; Wang, H.; Ye, J.; Li, F.; Zhang, Q.; Nabi, M. Benefit of solid-liquid separation on volatile fatty acid production from grass clipping with ultrasound-calcium hydroxide pretreatment. Bioresour. Technol. 2019, 274, 97–104. [Google Scholar] [CrossRef]
- Wan, J.; Fang, W.; Zhang, T.; Wen, G. Enhancement of fermentative volatile fatty acids production from waste activated sludge by combining sodium dodecylbenzene sulfonate and low-thermal pretreatment. Bioresour. Technol. 2020, 308, 123291. [Google Scholar] [CrossRef]
- Baeyens, J.; Zhang, H.; Nie, J.; Appels, L.; Dewil, R.; Ansart, R.; Deng, Y. Reviewing the potential of bio-hydrogen production by fermentation. Renew. Sustain. Energy Rev. 2020, 131, 110023. [Google Scholar] [CrossRef]
- Elsharnouby, O.; Hafez, H.; Nakhla, G.; El Naggar, M.H. A critical literature review on biohydrogen production by pure cultures. Int. J. Hydrogen Energy 2013, 38, 4945–4966. [Google Scholar] [CrossRef]
- Krupp, M.; Widmann, R. Biohydrogen production by dark fermentation: Experiences of continuous operation in large lab scale. Int. J. Hydrogen Energy 2009, 34, 4509–4516. [Google Scholar] [CrossRef]
- Mosey, F.E. Mathematical Modelling of the Anaerobic Digestion Process: Regulatory Mechanisms for the Forma Tion of Short-Chain Volatile Acids From Glucose; IWA: Nanjing, China, 1983; pp. 209–232. [Google Scholar]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Fernandez-Feito, R.; Dinsdale, R.M.; Guwy, A.J. Recovery and enhanced yields of volatile fatty acids from a grass fermentation via in-situ solids separation and electrodialysis. J. Clean. Prod. 2021, 296, 126430. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Short-chain fatty acids and hydrogen production in one single anaerobic fermentation stage using carbohydrate-rich food waste. J. Clean. Prod. 2021, 284, 124727. [Google Scholar] [CrossRef]
- Van Ginkel, S.; Logan, B.E. Inhibition of biohydrogen production by undissociated acetic and butyric acids. Environ. Sci. Technol. 2005, 39, 9351–9356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, Y.; Dai, K.; Shen, N.; Zeng, R.J. The glucose metabolic distribution in thermophilic (55 °C) mixed culture fermentation: A chemostat study. Int. J. Hydrogen Energy 2015, 40, 919–926. [Google Scholar] [CrossRef]
- Kirli, B.; Karapinar, I. The effect of HRT on biohydrogen production from acid hydrolyzed waste wheat in a continuously operated packed bed reactor. Int. J. Hydrogen Energy 2018, 43, 10678–10685. [Google Scholar] [CrossRef]
- Nagarajan, S.; Prasad Sarvothaman, V.; Knörich, M.; Ranade, V.V. A simplified model for simulating anaerobic digesters: Application to valorisation of bagasse and distillery spent wash. Bioresour. Technol. 2021, 337, 125395. [Google Scholar] [CrossRef]
- Kumar, G.; Sivagurunathan, P.; Park, J.H.; Park, J.H.; Park, H.D.; Yoon, J.J.; Kim, S.H. HRT dependent performance and bacterial community population of granular hydrogen-producing mixed cultures fed with galactose. Bioresour. Technol. 2016, 206, 188–194. [Google Scholar] [CrossRef]
- Massanet-Nicolau, J.; Dinsdale, R.; Guwy, A. Hydrogen production from sewage sludge using mixed microflora inoculum: Effect of pH and enzymatic pretreatment. Bioresour. Technol. 2008, 99, 6325–6331. [Google Scholar] [CrossRef]
- Moreno-Andrade, I.; Carrillo-Reyes, J.; Santiago, S.G.; Bujanos-Adame, M.C. Biohydrogen from food waste in a discontinuous process: Effect of HRT and microbial community analysis. J. Hydrogen Energy 2015, 30, 17246–17252. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Liu, Y.; Nghiem, L.D.; Chang, S.W.; Nguyen, D.D.; Zhang, S.; Luo, G.; Jia, H. Optimization of hydraulic retention time and organic loading rate for volatile fatty acid production from low strength wastewater in an anaerobic membrane bioreactor. Bioresour. Technol. 2019, 271, 100–108. [Google Scholar] [CrossRef]
- Lv, N.; Cai, G.; Pan, X.; Li, Y.; Wang, R.; Li, J.; Li, C.; Zhu, G. pH and hydraulic retention time regulation for anaerobic fermentation: Focus on volatile fatty acids production/distribution, microbial community succession and interactive correlation. Bioresour. Technol. 2022, 347, 126310. [Google Scholar] [CrossRef] [PubMed]
- Moretto, G.; Valentino, F.; Pavan, P.; Majone, M.; Bolzonella, D. Optimization of urban waste fermentation for volatile fatty acids production. Waste Manag. 2019, 92, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.; Duber, A.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Conversion of organic waste into volatile fatty acids–The influence of process operating parameters. Chem. Eng. J. 2018, 345, 395–403. [Google Scholar] [CrossRef]
- Ferraz Júnior, A.Ô.D.N.; Zaiat, M.; Gupta, M.; Elbeshbishy, E.; Hafez, H.; Nakhla, G. Impact of organic loading rate on biohydrogen production in an up-flow anaerobic packed bed reactor (UAnPBR). Bioresour. Technol. 2014, 164, 371–379. [Google Scholar] [CrossRef]
- Fuess, L.T.; Mazine Kiyuna, L.S.; Garcia, M.L.; Zaiat, M. Operational strategies for long-term biohydrogen production from sugarcane stillage in a continuous acidogenic packed-bed reactor. Int. J. Hydrogen Energy 2016, 41, 8132–8145. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Wang, X.; Hu, Y.; Zhang, Y.; Li, Y. Lactic acid fermentation from food waste with indigenous microbiota: Effects of pH, temperature and high OLR. Waste Manag. 2016, 52, 278–285. [Google Scholar] [CrossRef]
- Iglesias-Iglesias, R.; Campanaro, S.; Treu, L.; Kennes, C.; Veiga, M.C. Valorization of sewage sludge for volatile fatty acids production and role of microbiome on acidogenic fermentation. Bioresour. Technol. 2019, 291, 121817. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Greses, S.; González-Fernández, C. Impact of Organic Loading Rate in Volatile Fatty Acids Production and Population Dynamics Using Microalgae Biomass as Substrate. Sci. Rep. 2019, 9, 18374. [Google Scholar] [CrossRef]
- Zulkhairi, M.; Yusoff, M.; Rahman, N.a.A.; Abd-Aziz, S.; Ling, C.M.; Hassan, M.A.; Shirai, Y. The Effect of Hydraulic Retention Time and Volatile Fatty Acids on Biohydrogen Production from Palm Oil Mill Effluent under Non-Sterile Condition. Aust. J. Basic Appl. Sci. 2010, 4, 577–587. [Google Scholar]
- Rincón, B.; Sánchez, E.; Raposo, F.; Borja, R.; Travieso, L.; Martín, M.A.; Martín, A. Effect of the organic loading rate on the performance of anaerobic acidogenic fermentation of two-phase olive mill solid residue. Waste Manag. 2008, 28, 870–877. [Google Scholar] [CrossRef]
- Malinowsky, C.; Nadaleti, W.; Debiasi, L.R.; Gonçalves Moreira, A.J.; Bayard, R.; Borges de Castilhos Junior, A. Start-up phase optimization of two-phase anaerobic digestion of food waste: Effects of organic loading rate and hydraulic retention time. J. Environ. Manag. 2021, 296, 113064. [Google Scholar] [CrossRef] [PubMed]
- De Groof, V.; Coma, M.; Arnot, T.C.; Leak, D.J.; Lanham, A.B. Adjusting Organic Load as a Strategy to Direct Single-Stage Food Waste Fermentation from Anaerobic Digestion to Chain Elongation. Processes 2020, 8, 1487. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Karakashev, D.; Xie, L.; Zhou, Q.; Angelidaki, I. Long-term effect of inoculum pretreatment on fermentative hydrogen production by repeated batch cultivations: Homoacetogenesis and methanogenesis as competitors to hydrogen production. Biotechnol. Bioeng. 2011, 108, 1816–1827. [Google Scholar] [CrossRef]
- Chinellato, G.; Cavinato, C.; Bolzonella, D.; Heaven, S.; Banks, C.J. Biohydrogen production from food waste in batch and semi-continuous conditions: Evaluation of a two-phase approach with digestate recirculation for pH control. Int. J. Hydrogen Energy 2013, 38, 4351–4360. [Google Scholar] [CrossRef] [Green Version]
- Doi, T.; Matsumoto, H.; Abe, J.; Morita, S. Feasibility study on the application of rhizosphere microflora of rice for the biohydrogen production from wasted bread. Int. J. Hydrogen Energy 2009, 34, 1735–1743. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, S.K.; Shin, H.S. Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int. J. Hydrogen Energy 2004, 29, 1607–1616. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Kornaros, M. Effect of pH on the anaerobic acidogenesis of agroindustrial wastewaters for maximization of bio-hydrogen production: A lab-scale evaluation using batch tests. Bioresour. Technol. 2014, 162, 218–227. [Google Scholar] [CrossRef]
- Yokoyama, H.; Waki, M.; Moriya, N.; Yasuda, T.; Tanaka, Y.; Haga, K. Effect of fermentation temperature on hydrogen production from cow waste slurry by using anaerobic microflora within the slurry. Appl. Microbiol. Biotechnol. 2007, 74, 474–483. [Google Scholar] [CrossRef]
- Zhang, M.L.; Fan, Y.T.; Xing, Y.; Pan, C.M.; Zhang, G.S.; Lay, J.J. Enhanced biohydrogen production from cornstalk wastes with acidification pretreatment by mixed anaerobic cultures. Biomass Bioenergy 2007, 31, 250–254. [Google Scholar] [CrossRef]
- Tsigkou, K.; Tsafrakidou, P.; Athanasopoulou, S.; Zafiri, C.; Kornaros, M. Effect of pH on the Anaerobic Fermentation of Fruit/Vegetables and Disposable Nappies Hydrolysate for Bio-hydrogen Production. Waste Biomass Valorization 2020, 11, 539–551. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrre, H.; Steyer, J.P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Cabrera, F.; Serrano, A.; Torres, Á.; Rodriguez-Gutierrez, G.; Jeison, D.; Fermoso, F.G. The accumulation of volatile fatty acids and phenols through a pH-controlled fermentation of olive mill solid waste. Sci. Total Environ. 2019, 657, 1501–1507. [Google Scholar] [CrossRef]
- Jankowska, E.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Volatile fatty acids production during mixed culture fermentation–The impact of substrate complexity and pH. Chem. Eng. J. 2017, 326, 901–910. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Q.; Wang, X.; Zhou, X.; Zhu, J. Effect of pH on volatile fatty acid production from anaerobic digestion of potato peel waste. Bioresour. Technol. 2020, 316, 123851. [Google Scholar] [CrossRef]
- Ye, M.; Luo, J.; Zhang, S.; Yang, H.; Li, Y.-Y.; Liu, J. In-situ ammonia stripping with alkaline fermentation of waste activated sludge to improve short-chain fatty acids production and carbon source availability. Bioresour. Technol. 2020, 301, 122782. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Schnürer, A.; Cetecioglu, Z. Volatile fatty acids production via mixed culture fermentation: Revealing the link between pH, inoculum type and bacterial composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef]
- Begum, S.; Anupoju, G.R.; Sridhar, S.; Bhargava, S.K.; Jegatheesan, V.; Eshtiaghi, N. Evaluation of single and two stage anaerobic digestion of landfill leachate: Effect of pH and initial organic loading rate on volatile fatty acid (VFA) and biogas production. Bioresour. Technol. 2018, 251, 364–373. [Google Scholar] [CrossRef]
- Nguyen, P.K.T.; Kim, J.; Das, G.; Yoon, H.H.; Lee, D.H. Optimization of simultaneous dark fermentation and microbial electrolysis cell for hydrogen production from macroalgae using response surface methodology. Biochem. Eng. J. 2021, 171, 108029. [Google Scholar] [CrossRef]
- Connaughton, S.; Collins, G.; O’Flaherty, V. Psychrophilic and mesophilic anaerobic digestion of brewery effluent: A comparative study. Water Res. 2006, 40, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.R.; Rouissi, T.; Brar, S.K.; Surampalli, R.Y. Critical insights into psychrophilic anaerobic digestion: Novel strategies for improving biogas production. Waste Manag. 2021, 131, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, J.; Huang, R.; Zhang, W.; He, W.; Deng, Z.; Han, Y.; Xiao, B.; Luo, H.; Qu, W. Effects of temperature and total solid content on biohydrogen production from dark fermentation of rice straw: Performance and microbial community characteristics. Chemosphere 2022, 286, 131655. [Google Scholar] [CrossRef]
- Karlsson, A.; Vallin, L.; Ejlertsson, J. Effects of temperature, hydraulic retention time and hydrogen extraction rate on hydrogen production from the fermentation of food industry residues and manure. Int. J. Hydrogen Energy 2008, 33, 953–962. [Google Scholar] [CrossRef]
- Zheng, H.-S.; Guo, W.-Q.; Yang, S.-S.; Feng, X.-C.; Du, J.-S.; Zhou, X.-J.; Chang, J.-S.; Ren, N.-Q. Thermophilic hydrogen production from sludge pretreated by thermophilic bacteria: Analysis of the advantages of microbial community and metabolism. Bioresour. Technol. 2014, 172, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Ziara, R.M.M.; Miller, D.N.; Subbiah, J.; Dvorak, B.I. Lactate wastewater dark fermentation: The effect of temperature and initial pH on biohydrogen production and microbial community. Int. J. Hydrogen Energy 2019, 44, 661–673. [Google Scholar] [CrossRef]
- Huang, C.; Wang, W.; Sun, X.; Shen, J.; Wang, L. A novel acetogenic bacteria isolated from waste activated sludge and its potential application for enhancing anaerobic digestion performance. J. Environ. Manag. 2020, 255, 109842. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Hjort, M.; Cirne, D.; Gérardin, F.; Lacroix, S.; Gaval, G.; Karabegovic, L.; Alexandersson, T.; Johansson, P.; Karlsson, A.; et al. Integrated production of polyhydroxyalkanoates (PHAs) with municipal wastewater and sludge treatment at pilot scale. Bioresour. Technol. 2015, 181, 78–89. [Google Scholar] [CrossRef]
- Garcia-Aguirre, J.; Aymerich, E.; de Goñi, J.G.-M.; Esteban-Gutiérrez, M. Selective VFA production potential from organic waste streams: Assessing temperature and pH influence. Bioresour. Technol. 2017, 244, 1081–1088. [Google Scholar] [CrossRef]
- Xiao, B.; Qin, Y.; Wu, J.; Chen, H.; Yu, P.; Liu, J.; Li, Y.Y. Comparison of single-stage and two-stage thermophilic anaerobic digestion of food waste: Performance, energy balance and reaction process. Energy Convers. Manag. 2018, 156, 215–223. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Tan, T. Batch and semi-continuous anaerobic digestion of food waste in a dual solid-liquid system. Bioresour. Technol. 2013, 145, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Show, K.Y.; Tay, J.H.; Liang, D.T.; Lee, D.J. Biohydrogen production with anaerobic fluidized bed reactors-A comparison of biofilm-based and granule-based systems. Int. J. Hydrogen Energy 2008, 33, 1559–1564. [Google Scholar] [CrossRef]
- Brar, K.K.; Cortez, A.A.; Pellegrini, V.O.A.; Amulya, K.; Polikarpov, I.; Magdouli, S.; Kumar, M.; Yang, Y.-H.; Bhatia, S.K.; Brar, S.K. An overview on progress, advances, and future outlook for biohydrogen production technology. Int. J. Hydrogen Energy 2022, in press. [Google Scholar] [CrossRef]
- Castillo-Hernández, A.; Mar-Alvarez, I.; Moreno-Andrade, I. Start-up and operation of continuous stirred-tank reactor for biohydrogen production from restaurant organic solid waste. Int. J. Hydrogen Energy 2015, 2015, 17239–17245. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y. Coproduction of hydrogen and methane in a CSTR-IC two-stage anaerobic digestion system from molasses wastewater. Water Sci. Technol. 2019, 79, 270–277. [Google Scholar] [CrossRef]
- Ri, P.C.; Kim, J.S.; Kim, T.R.; Pang, C.H.; Mun, H.G.; Pak, G.C.; Ren, N.Q. Effect of hydraulic retention time on the hydrogen production in a horizontal and vertical continuous stirred-tank reactor. Int. J. Hydrogen Energy 2019, 44, 17742–17749. [Google Scholar] [CrossRef]
- Tena, M.; Perez, M.; Solera, R. Effect of hydraulic retention time on hydrogen production from sewage sludge and wine vinasse in a thermophilic acidogenic CSTR: A promising approach for hydrogen production within the biorefinery concept. Int. J. Hydrogen Energy 2021, 46, 7810–7820. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejó, E.; Gónzalez-Fernández, C. Agroindustrial waste as a resource for volatile fatty acids production via anaerobic fermentation. Bioresour. Technol. 2020, 297, 122486. [Google Scholar] [CrossRef]
- Jones, R.J.; Fernández-feito, R.; Massanet-nicolau, J.; Dinsdale, R.; Guwy, A. Continuous recovery and enhanced yields of volatile fatty acids from a continually-fed 100 L food waste bioreactor by filtration and electrodialysis. Waste Manag. 2021, 122, 81–88. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Shanmuganathan, R.; Pugazhendhi, A.; Gunasekar, P.; Manigandan, S. Biohydrogen production using horizontal and vertical continuous stirred tank reactor- a numerical optimization. Int. J. Hydrogen Energy 2021, 46, 11305–11312. [Google Scholar] [CrossRef]
- Keskin, T.; Giusti, L.; Azbar, N. Continuous biohydrogen production in immobilized biofilm system versus suspended cell culture. Int. J. Hydrogen Energy 2012, 37, 1418–1424. [Google Scholar] [CrossRef]
- Anjana Anand, A.S.; Adish Kumar, S.; Rajesh Banu, J.; Ginni, G. The performance of fluidized bed solar photo Fenton oxidation in the removal of COD from hospital wastewaters. Desalin. Water Treat. 2016, 57, 8236–8242. [Google Scholar] [CrossRef]
- Chaves, T.C.; Gois, G.N.S.B.; Peiter, F.S.; Vich, D.V.; de Amorim, E.L.C. Biohydrogen production in an AFBR using sugarcane molasses. Bioprocess. Biosyst. Eng. 2021, 44, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tay, J.; Show, K.; Yan, R.; Teeliang, D.; Lee, D.; Jiang, W. Biohydrogen production in a granular activated carbon anaerobic fluidized bed reactor. Int. J. Hydrogen Energy 2007, 32, 185–191. [Google Scholar] [CrossRef]
- Barros, A.R.; Cavalcante de Amorim, E.L.; Reis, C.M.; Shida, G.M.; Silva, E.L. Biohydrogen production in anaerobic fluidized bed reactors: Effect of support material and hydraulic retention time. Int. J. Hydrogen Energy 2010, 35, 3379–3388. [Google Scholar] [CrossRef]
- Amorim, N.C.S.; Alves, I.; Martins, J.S.; Amorim, E.L.C. Biohydrogen production from cassava wastewater in an anaerobic fluidized bed reactor. Braz. J. Chem. Eng. 2014, 31, 603–612. [Google Scholar] [CrossRef]
- Maaroff, R.M.; Md Jahim, J.; Azahar, A.M.; Abdul, P.M.; Masdar, M.S.; Nordin, D.; Abd Nasir, M.A. Biohydrogen production from palm oil mill effluent (POME) by two stage anaerobic sequencing batch reactor (ASBR) system for better utilization of carbon sources in POME. Int. J. Hydrogen Energy 2019, 44, 3395–3406. [Google Scholar] [CrossRef]
- Santiago, S.G.; Morgan-Sagastume, J.M.; Monroy, O.; Moreno-Andrade, I. Biohydrogen production from organic solid waste in a sequencing batch reactor: An optimization of the hydraulic and solids retention time. Int. J. Hydrogen Energy 2020, 45, 25681–25688. [Google Scholar] [CrossRef]
- Lagoa-Costa, B.; Kennes, C.; Veiga, M.C. Cheese whey fermentation into volatile fatty acids in an anaerobic sequencing batch reactor. Bioresour. Technol. 2020, 308, 123226. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Tay, J.H.; Lin, C.Y.; Chang, J.S. Biohydrogen production: Current perspectives and the way forward. Int. J. Hydrogen Energy 2012, 37, 15616–15631. [Google Scholar] [CrossRef]
- Anzola-Rojas, M.d.P.; Gonçalves da Fonseca, S.; Canedo da Silva, C.; Maia de Oliveira, V.; Zaiat, M. The use of the carbon/nitrogen ratio and specific organic loading rate as tools for improving biohydrogen production in fixed-bed reactors. Biotechnol. Rep. 2015, 5, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomczak, W.; Ferrasse, J.-H.; Giudici-Orticoni, M.-T.; Soric, A. Effect of hydraulic retention time on a continuous biohydrogen production in a packed bed biofilm reactor with recirculation flow of the liquid phase. Int. J. Hydrogen Energy 2018, 43, 18883–18895. [Google Scholar] [CrossRef] [Green Version]
- Muri, P.; Marinšek-Logar, R.; Djinović, P.; Pintar, A. Influence of support materials on continuous hydrogen production in anaerobic packed-bed reactor with immobilized hydrogen producing bacteria at acidic conditions. Enzyme Microb. Technol. 2018, 111, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.A.; Ghufran, R.; Wahid, Z.A.; Ahmad, A. Integrated application of upflow anaerobic sludge blanket reactor for the treatment of wastewaters. Water Res. 2011, 45, 4683–4699. [Google Scholar] [CrossRef]
- Castelló, E.; García y Santos, C.; Iglesias, T.; Paolino, G.; Wenzel, J.; Borzacconi, L.; Etchebehere, C. Feasibility of biohydrogen production from cheese whey using a UASB reactor: Links between microbial community and reactor performance. Int. J. Hydrogen Energy 2009, 34, 5674–5682. [Google Scholar] [CrossRef]
- Eregowda, T.; Kokko, M.E.; Rene, E.R.; Rintala, J.; Lens, P.N.L. Volatile fatty acid production from Kraft mill foul condensate in upflow anaerobic sludge blanket reactors. Environ. Technol. 2021, 42, 2447–2460. [Google Scholar] [CrossRef]
- Intanoo, P.; Chaimongkol, P.; Chavadej, S. Hydrogen and methane production from cassava wastewater using two-stage upflow anaerobic sludge blanket reactors (UASB) with an emphasis on maximum hydrogen production. Int. J. Hydrogen Energy 2016, 41, 6107–6114. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, D.-H.; Shin, H.-S. Continuous fermentative hydrogen production from coffee drink manufacturing wastewater by applying UASB reactor. Int. J. Hydrogen Energy 2010, 35, 13370–13378. [Google Scholar] [CrossRef]
- Yang, H.; Shao, P.; Lu, T.; Shen, J.; Wang, D.; Xu, Z.; Yuan, X. Continuous bio-hydrogen production from citric acid wastewater via facultative anaerobic bacteria. Int. J. Hydrogen Energy 2006, 31, 1306–1313. [Google Scholar] [CrossRef]
- Estevez, M.M.; Linjordet, R.; Morken, J. Effects of steam explosion and co-digestion in the methane production from Salix by mesophilic batch assays. Bioresour. Technol. 2012, 104, 749–756. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F.; Kapdan, I.K.; Oztekin, R. Biohydrogen production by dark fermentation of wheat powder solution: Effects of C/N and C/P ratio on hydrogen yield and formation rate. Int. J. Hydrogen Energy 2008, 33, 1813–1819. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lay, C.H. Carbon/nitrogen-ratio effect on fermentative hydrogen production by mixed microflora. Int. J. Hydrogen Energy 2004, 29, 41–45. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Yang, G.; Sun, G.; Sage, V. A Review of the Enhancement of Bio-Hydrogen Generation by Chemicals Addition. Catalysts 2019, 9, 353. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, Y.; Yang, G.; Sun, Z. Optimization of biohydrogen production using acid pretreated corn stover hydrolysate followed by nickel nanoparticle addition. Int. J. Energy Res. 2020, 44, 1843–1857. [Google Scholar] [CrossRef]
- Sinharoy, A.; Pakshirajan, K. A novel application of biologically synthesized nanoparticles for enhanced biohydrogen production and carbon monoxide bioconversion. Renew. Energy 2020, 147, 864–873. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Tian, Y.; Zheng, L.; Hao, H.; Huang, H. Impact of Fe and Ni Addition on the VFAs’ Generation and Process Stability of Anaerobic Fermentation Containing Cd. Int. J. Environ. Res. Public Health 2019, 16, 4066. [Google Scholar] [CrossRef] [Green Version]
- Dahiya, S.; Lakshminarayanan, S.; Venkata Mohan, S. Steering acidogenesis towards selective propionic acid production using co-factors and evaluating environmental sustainability. Chem. Eng. J. 2020, 379, 122135. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, C.; Ren, H.-Y.; Wu, J.-T.; Meng, J.; Nan, J.; Cao, G.-L.; Yang, S.-S.; Ren, N.-Q. Feasibility of enhancing hydrogen production from cornstalk hydrolysate anaerobic fermentation by RCPH-biochar. Bioresour. Technol. 2020, 297, 122505. [Google Scholar] [CrossRef]
- Sugiarto, Y.; Sunyoto, N.M.S.; Zhu, M.; Jones, I.; Zhang, D. Effect of biochar in enhancing hydrogen production by mesophilic anaerobic digestion of food wastes: The role of minerals. Int. J. Hydrogen Energy 2021, 46, 3695–3703. [Google Scholar] [CrossRef]
- Lu, J.-H.; Chen, C.; Huang, C.; Zhuang, H.; Leu, S.-Y.; Lee, D.-J. Dark fermentation production of volatile fatty acids from glucose with biochar amended biological consortium. Bioresour. Technol. 2020, 303, 122921. [Google Scholar] [CrossRef]
- Taheri, E.; Amin, M.M.; Fatehizadeh, A.; Pourzamani, H.; Bina, B.; Spanjers, H. Biohydrogen production under hyper salinity stress by an anaerobic sequencing batch reactor with mixed culture. J. Environ. Health Sci. Eng. 2018, 16, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, O.; Kiran Katari, J.; Chatterjee, S.; Venkata Mohan, S. Salinity induced acidogenic fermentation of food waste regulates biohydrogen production and volatile fatty acids profile. Fuel 2020, 276, 117794. [Google Scholar] [CrossRef]
- He, X.; Yin, J.; Liu, J.; Chen, T.; Shen, D. Characteristics of acidogenic fermentation for volatile fatty acid production from food waste at high concentrations of NaCl. Bioresour. Technol. 2019, 271, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, X.; Chen, F.; Wang, Y.; Li, X.; Wang, D.; Tao, Z.; Xu, D.; Xue, W.; Geng, M.; et al. Clarithromycin affect methane production from anaerobic digestion of waste activated sludge. J. Clean. Prod. 2020, 255, 120321. [Google Scholar] [CrossRef]
- Huang, X.; Xu, Q.; Wu, Y.; Wang, D.; Yang, Q.; Chen, F.; Wu, Y.; Pi, Z.; Chen, Z.; Li, X.; et al. Effect of clarithromycin on the production of volatile fatty acids from waste activated sludge anaerobic fermentation. Bioresour. Technol. 2019, 288, 121598. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, X.; Zhou, Y.; Yang, X.; Lam, S.S.; Wang, D. Influence of roxithromycin as antibiotic residue on volatile fatty acids recovery in anaerobic fermentation of waste activated sludge. J. Hazard. Mater. 2020, 394, 122570. [Google Scholar] [CrossRef]
- Tao, Z.; Yang, Q.; Yao, F.; Huang, X.; Wu, Y.; Du, M.; Chen, S.; Liu, X.; Li, X.; Wang, D. The inhibitory effect of thiosulfinate on volatile fatty acid and hydrogen production from anaerobic co-fermentation of food waste and waste activated sludge. Bioresour. Technol. 2020, 297, 122428. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Mulder, M.J.J.; Premier, G.; Dinsdale, R.; Guwy, A. Increased biohydrogen yields, volatile fatty acid production and substrate utilisation rates via the electrodialysis of a continually fed sucrose fermenter. Bioresour. Technol. 2017, 229, 46–52. [Google Scholar] [CrossRef]
- Hassan, G.K.; Jones, R.J.; Massanet-Nicolau, J.; Dinsdale, R.; Abo-Aly, M.M.; El-Gohary, F.A.; Guwy, A. Increasing 2 -Bio- (H2 and CH4) production from food waste by combining two-stage anaerobic digestion and electrodialysis for continuous volatile fatty acids removal. Waste Manag. 2021, 129, 20–25. [Google Scholar] [CrossRef]
- Bundhoo, M.A.Z.; Mohee, R. Inhibition of dark fermentative bio-hydrogen production: A review. Int. J. Hydrogen Energy 2016, 41, 6713–6733. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Astals, S.; Robles, Á.; Steyer, J.P. Unraveling the literature chaos around free ammonia inhibition in anaerobic digestion. Renew. Sustain. Energy Rev. 2020, 117, 109487. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.; Tatoulis, T.; Akratos, C.; Tekerlekopoulou, A.; Vayenas, D. Zeolite as a Potential Medium for Ammonium Recovery and Second Cheese Whey Treatment. Water 2019, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Chen, F.; Xue, S.; Pan, J.; Khoshnevisan, B.; Yang, Y.; Liu, H.; Qiu, L. Improving anaerobic digestion of chicken manure under optimized biochar supplementation strategies. Bioresour. Technol. 2021, 325, 124697. [Google Scholar] [CrossRef]
- Ho, L.; Ho, G. Mitigating ammonia inhibition of thermophilic anaerobic treatment of digested piggery wastewater: Use of pH reduction, zeolite, biomass and humic acid. Water Res. 2012, 46, 4339–4350. [Google Scholar] [CrossRef] [Green Version]
- Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Ross, A.B. Influence of augmentation of biochar during anaerobic co-digestion of Chlorella vulgaris and cellulose. Bioresour. Technol. 2022, 343, 126086. [Google Scholar] [CrossRef]
- Silva, R.M.; Abreu, A.A.; Salvador, A.F.; Alves, M.M.; Neves, I.C.; Pereira, M.A. Zeolite addition to improve biohydrogen production from dark fermentation of C5/C6-sugars and Sargassum sp. biomass. Sci. Rep. 2021, 11, 16350. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Choi, J.-A.; Oh, Y.-K.; Abou-Shanab, R.A.I.; Song, H.; Min, B.; Cho, Y.; Na, J.-G.; Koo, J.; Jeon, B.-H. Hydrogen production from sulfate- and ferrous-enriched wastewater. Int. J. Hydrogen Energy 2011, 36, 13984–13990. [Google Scholar] [CrossRef]
- Mizuno, O.; Li, Y.Y.; Noike, T. The behavior of sulfate-reducing bacteria in acidogenic phase of anaerobic digestion. Water Res. 1998, 32, 1626–1634. [Google Scholar] [CrossRef]
- Haosagul, S.; Prommeenate, P.; Hobbs, G.; Pisutpaisal, N. Sulfide-oxidizing bacteria community in full-scale bioscrubber treating H2S in biogas from swine anaerobic digester. Renew. Energy 2020, 150, 973–980. [Google Scholar] [CrossRef]
- Kobayashi, T.; Li, Y.-Y.; Kubota, K.; Harada, H.; Maeda, T.; Yu, H.-Q. Characterization of sulfide-oxidizing microbial mats developed inside a full-scale anaerobic digester employing biological desulfurization. Appl. Microbiol. Biotechnol. 2012, 93, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Dhar, B.R.; Elbeshbishy, E.; Nakhla, G. Influence of iron on sulfide inhibition in dark biohydrogen fermentation. Bioresour. Technol. 2012, 126, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hao, X.; van Loosdrecht, M.C.M.; Liu, R. Relieving the inhibition of humic acid on anaerobic digestion of excess sludge by metal ions. Water Res. 2021, 188, 116541. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cheng, J.; Ding, L.; Song, W.; Zhou, J.; Cen, K. Inhibitory effects of furan derivatives and phenolic compounds on dark hydrogen fermentation. Bioresour. Technol. 2015, 196, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Reyes, Á.; Cubero-Cardoso, J.; Rodríguez-Gutiérrez, G.; García-Martín, J.F.; Rodríguez-Galán, M.; Borja, R.; Serrano, A.; Fermoso, F.G. Extraction of phenolic compounds and production of biomethane from strawberry and raspberry extrudates. Biochem. Eng. J. 2019, 147, 11–19. [Google Scholar] [CrossRef]
- Dong, D.; Wang, R.; Geng, P.; Li, C.; Zhao, Z. Enhancing effects of activated carbon supported nano zero-valent iron on anaerobic digestion of phenol-containing organic wastewater. J. Environ. Manag. 2019, 244, 1–12. [Google Scholar] [CrossRef]
- Kim, J.R.; Karthikeyan, K.G. Effects of severe pretreatment conditions and lignocellulose-derived furan byproducts on anaerobic digestion of dairy manure. Bioresour. Technol. 2021, 340, 125632. [Google Scholar] [CrossRef]
- Wijayasekera, S.C.; Hewage, K.; Siddiqui, O.; Hettiaratchi, P.; Sadiq, R. Waste-to-hydrogen technologies: A critical review of techno-economic and socio-environmental sustainability. Int. J. Hydrogen Energy 2022, 47, 5842–5870. [Google Scholar] [CrossRef]
- Massanet-Nicolau, J.; Jones, R.J.; Guwy, A.; Dinsdale, R.; Premier, G.; Mulder, M.J.J. Maximising Biohydrogen Yields via Continuous Electrochemical Hydrogen Removal and Carbon Dioxide Scrubbing. Bioresour. Technol. 2016, 218, 512–517. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Fernandez–Feito, R.; Dinsdale, R.M.; Guwy, A.J. Fermentative volatile fatty acid production and recovery from grass using a novel combination of solids separation, pervaporation, and electrodialysis technologies. Bioresour. Technol. 2021, 342, 125926. [Google Scholar] [CrossRef]
- Jones, R.; Massanet-Nicolau, J.; Guwy, A. A review of carboxylate production and recovery from organic wastes. Bioresour. Technol. Rep. 2021, 16, 100826. [Google Scholar]
- Scoma, A.; Varela-Corredor, F.; Bertin, L.; Gostoli, C.; Bandini, S. Recovery of VFAs from anaerobic digestion of dephenolized Olive Mill Wastewaters by Electrodialysis. Sep. Purif. Technol. 2016, 159, 81–91. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- IEA. The Future of Hydrogen; IEA: Paris, France, 2019. [Google Scholar]
- Maestre, V.M.; Ortiz, A.; Ortiz, I. Challenges and prospects of renewable hydrogen-based strategies for full decarbonization of stationary power applications. Renew. Sustain. Energy Rev. 2021, 152, 111628. [Google Scholar] [CrossRef]
- International Renewable Energy Agency. Renewable Capacity Statistics 2020 Statistiques De Capacité Renouvelable 2020 Estadísticas De Capacidad Renovable 2020. 2020. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2020/Mar/IRENA_RE_Capacity_Statistics_2020.pdf (accessed on 8 April 2022).
- International Renewable Energy Agency. 2050 ENERGY TRANSFORMATION E D I T I O N : 2 0 2 0 GLOBAL RENEWABLES OUTLOOK. 2020. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2020/Apr/IRENA_Global_Renewables_Outlook_2020.pdf (accessed on 8 April 2022).
- Janssen, J.L.L.C.C.; Weeda, M.; Detz, R.J.; van der Zwaan, B. Country-specific cost projections for renewable hydrogen production through off-grid electricity systems. Appl. Energy 2022, 309, 118398. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Shanmugam, S.; Sekar, M.; Mathimani, T.; Incharoensakdi, A.; Kim, S.H.; Parthiban, A.; Edwin Geo, V.; Brindhadevi, K.; Pugazhendhi, A. Insights on biological hydrogen production routes and potential microorganisms for high hydrogen yield. Fuel 2021, 291, 120136. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, D.; Li, X.; Yang, Q.; Xu, Q.; Ni, B.J.; Wang, Q.; Liu, X. Towards hydrogen production from waste activated sludge: Principles, challenges and perspectives. Renew. Sustain. Energy Rev. 2021, 135, 110283. [Google Scholar] [CrossRef]
- Park, J.H.; Chandrasekhar, K.; Jeon, B.H.; Jang, M.; Liu, Y.; Kim, S.H. State-of-the-art technologies for continuous high-rate biohydrogen production. Bioresour. Technol. 2021, 320, 124304. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Kavitha, S.; Preethi; Parthiba Karthikeyan, O.; Kumar, G.; Dai-Viet, N.V.; Rajesh Banu, J. Techno-economic assessment of various hydrogen production methods—A review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef]
- Habashy, M.M.; Ong, E.S.; Abdeldayem, O.M.; Al-Sakkari, E.G.; Rene, E.R. Food Waste: A Promising Source of Sustainable Biohydrogen Fuel. Trends Biotechnol. 2021, 39, 1274–1288. [Google Scholar] [CrossRef]
- Aydin, M.I.; Karaca, A.E.; Qureshy, A.M.M.I.; Dincer, I. A comparative review on clean hydrogen production from wastewaters. J. Environ. Manag. 2021, 279, 111793. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Wang, J. Review and comparison of various hydrogen production methods based on costs and life cycle impact assessment indicators. Int. J. Hydrogen Energy 2021, 46, 38612–38635. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Pal, A. An overview of conventional and non-conventional hydrogen production methods. Mater. Today Proc. 2021, 46, 5353–5359. [Google Scholar] [CrossRef]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-hydrogen: A review of main routes production, processes evaluation and techno-economical assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Duman, A.C.; Güler, Ö. Techno-economic analysis of off-grid PV/wind/fuel cell hybrid system combinations with a comparison of regularly and seasonally occupied households. Sustain. Cities Soc. 2018, 42, 107–126. [Google Scholar] [CrossRef]
- Dawood, F.; Shafiullah, G.M.; Anda, M. Stand-Alone Microgrid with 100% Renewable Energy: A Case Study with Hybrid Solar PV-Battery-Hydrogen. Sustainability 2020, 12, 2047. [Google Scholar] [CrossRef] [Green Version]
- Nascimento da Silva, G.; Rochedo, P.R.R.; Szklo, A. Renewable hydrogen production to deal with wind power surpluses and mitigate carbon dioxide emissions from oil refineries. Appl. Energy 2022, 311, 118631. [Google Scholar] [CrossRef]
- Nazir, H.; Muthuswamy, N.; Louis, C.; Jose, S.; Prakash, J.; Buan, M.E.M.; Flox, C.; Chavan, S.; Shi, X.; Kauranen, P.; et al. Is the H2 economy realizable in the foreseeable future? Part III: H2 usage technologies, applications, and challenges and opportunities. Int. J. Hydrogen Energy 2020, 45, 28217–28239. [Google Scholar] [CrossRef]
- Arora, A.; Zantye, M.S.; Hasan, M.M.F. Sustainable hydrogen manufacturing via renewable-integrated intensified process for refueling stations. Appl. Energy 2022, 311, 118667. [Google Scholar] [CrossRef]
- Symes, D.; Maillard, J.G.; Courtney, J.; Watton, J.; Meadowcroft, A.; Chandan, A.S.; Gurley, L.; Priestly, R.; Serdaroglu, G. Development of a hydrogen fuelling infrastructure in the Northeast U.S.A. Int. J. Hydrogen Energy 2014, 39, 7460–7466. [Google Scholar] [CrossRef]
- Schlund, D.; Schönfisch, M. Analysing the impact of a renewable hydrogen quota on the European electricity and natural gas markets. Appl. Energy 2021, 304, 117666. [Google Scholar] [CrossRef]
- Dolci, F.; Thomas, D.; Hilliard, S.; Guerra, C.F.; Hancke, R.; Ito, H.; Jegoux, M.; Kreeft, G.; Leaver, J.; Newborough, M.; et al. Incentives and legal barriers for power-to-hydrogen pathways: An international snapshot. Int. J. Hydrogen Energy 2019, 44, 11394–11401. [Google Scholar] [CrossRef]
- Veluswamy, G.K.; Shah, K.; Ball, A.S.; Guwy, A.J.; Dinsdale, R.M. A techno-economic case for volatile fatty acid production for increased sustainability in the wastewater treatment industry. Environ. Sci. Water Res. Technol. 2021, 7, 927–941. [Google Scholar] [CrossRef]
- Bahreini, G.; Elbeshbishy, E.; Jimenez, J.; Santoro, D.; Nakhla, G. Integrated fermentation and anaerobic digestion of primary sludges for simultaneous resource and energy recovery: Impact of volatile fatty acids recovery. Waste Manag. 2020, 118, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Moscariello, C.; Matassa, S.; Pirozzi, F.; Esposito, G.; Papirio, S. Valorisation of industrial hemp (Cannabis sativa L.) biomass residues through acidogenic fermentation and co-fermentation for volatile fatty acids production. Bioresour. Technol. 2022, 355, 127289. [Google Scholar] [CrossRef] [PubMed]
- Huq, N.A.; Hafenstine, G.R.; Huo, X.; Nguyen, H.; Tifft, S.M.; Conklin, D.R.; Stück, D.; Stunkel, J.; Yang, Z.; Heyne, J.S.; et al. Toward net-zero sustainable aviation fuel with wet waste–derived volatile fatty acids. Proc. Natl. Acad. Sci. USA 2021, 118, e2023008118. [Google Scholar] [CrossRef]
- Patterson, T.; Massanet-Nicolau, J.; Jones, R.; Boldrin, A.; Valentino, F.; Dinsdale, R.; Guwy, A. Utilizing grass for the biological production of polyhydroxyalkanoates (PHAs) via green biorefining: Material and energy flows. J. Ind. Ecol. 2020, 25, 802–815. [Google Scholar] [CrossRef]
- Kumar, G.; Ponnusamy, V.K.; Bhosale, R.R.; Shobana, S.; Yoon, J.-J.; Bhatia, S.K.; Rajesh Banu, J.; Kim, S.-H. A review on the conversion of volatile fatty acids to polyhydroxyalkanoates using dark fermentative effluents from hydrogen production. Bioresour. Technol. 2019, 287, 121427. [Google Scholar] [CrossRef]
- Policastro, G.; Giugliano, M.; Luongo, V.; Napolitano, R.; Fabbricino, M. Enhancing photo fermentative hydrogen production using ethanol rich dark fermentation effluents. Int. J. Hydrogen Energy 2022, 47, 117–126. [Google Scholar] [CrossRef]
- Cheng, J.; Li, H.; Ding, L.; Zhou, J.; Song, W.; Li, Y.-Y.; Lin, R. Improving hydrogen and methane co-generation in cascading dark fermentation and anaerobic digestion: The effect of magnetite nanoparticles on microbial electron transfer and syntrophism. Chem. Eng. J. 2020, 397, 125394. [Google Scholar] [CrossRef]





| Reactor Configuration | Advantages | Disadvantages |
|---|---|---|
| CSTR |
|
|
| AFBR |
|
|
| ASBR |
|
|
| APBR |
|
|
| UASBR |
|
|
| Recovery and Production Methods | Fermentation Conditions | Recovery and Production Data | Author |
|---|---|---|---|
| Hydrogen Recovery | |||
| Electrochemical proton exchange membrane and CO2 scrubbing of bioreactor gas phase; Homoacetogenesis and end-product inhibition arrested | Sucrose inoculated with heat-treated AD digestate. 3.34 L, continuously fed CSTR; pH 5.5; 35 °C; 24 h HRT | 1.79 mol H2/mol hexose 7 cmH23/min >99% purity of recovered H2 | [205] |
| As above with electrodialytic recovery of VFAs from the liquid phase to arrest end-product inhibition further | As above with 48 h HRT | 0.90 mol H2/mol hexose 3.47 cmH23/min >99% purity of recovered H2 | [184] |
| VFA Recovery | |||
| Filtration and electrodialysis for in situ VFA recovery; Methanogenesis and end-product inhibition arrested | 1% TS food waste inoculated with heat-treated AD digestate. 100 L continuously fed CSTR; pH 5.5; 35 °C, 10 d HRT | 17 g VFA/day recovered from bioreactor | [145] |
| Inline ultrasonic sieving, centrifugation, microfiltration, and electrodialysis for in situ VFA recovery; Methanogenesis and end product inhibition arrested | 5% TS grass waste inoculated with heat-treated AD digestate. 100 L continuously fed CSTR; pH 5.5; 35 °C, 8.25 d HRT | VFAs continually recovered into an external 30 L solution of up to 4500 mg VFA/L VFA yields of 404 mg VFA/g VS achieved | [87] |
| As above with an additional pervaporation stage before electrodialysis to aid VFA selectivity | As above with 7 d HRT | VFAs continually recovered into an external 30 L solution of up to 4000 mg VFA/L VFA yields of 875 mg VFA/g VS achieved | [206] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagarajan, S.; Jones, R.J.; Oram, L.; Massanet-Nicolau, J.; Guwy, A. Intensification of Acidogenic Fermentation for the Production of Biohydrogen and Volatile Fatty Acids—A Perspective. Fermentation 2022, 8, 325. https://doi.org/10.3390/fermentation8070325
Nagarajan S, Jones RJ, Oram L, Massanet-Nicolau J, Guwy A. Intensification of Acidogenic Fermentation for the Production of Biohydrogen and Volatile Fatty Acids—A Perspective. Fermentation. 2022; 8(7):325. https://doi.org/10.3390/fermentation8070325
Chicago/Turabian StyleNagarajan, Sanjay, Rhys Jon Jones, Lucy Oram, Jaime Massanet-Nicolau, and Alan Guwy. 2022. "Intensification of Acidogenic Fermentation for the Production of Biohydrogen and Volatile Fatty Acids—A Perspective" Fermentation 8, no. 7: 325. https://doi.org/10.3390/fermentation8070325
APA StyleNagarajan, S., Jones, R. J., Oram, L., Massanet-Nicolau, J., & Guwy, A. (2022). Intensification of Acidogenic Fermentation for the Production of Biohydrogen and Volatile Fatty Acids—A Perspective. Fermentation, 8(7), 325. https://doi.org/10.3390/fermentation8070325