Engineered Bacillus subtilis for the Production of Tetramethylpyrazine,(R,R)-2,3-Butanediol and Acetoin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, and Primers
2.2. Construction of Recombinant Strains and Validation of Enzyme Activity
2.3. Shake Flask Fermentation and Bioreactor Fermentation Methods
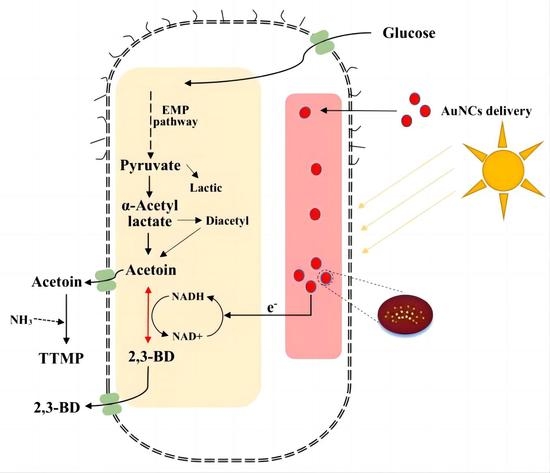
2.4. Construction and Fermentation of Engineered Photocatalytic System Bacteria
2.5. Analysis Method
3. Results and Discussion
3.1. Determination of Enzyme Activity and Optimization of Culture Conditions of Recombinant Strains
3.2. Production of 2,3-BD and AC by Batch Replenishment Fermentation with the BS-ppb11
3.3. Staged Batch Replenishment Fermentation of BS-ppb11 for TTMP Production
3.4. Light Fermentation of Engineered Photocatalytic System Bacteria BS-ppb12
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatti-Kaul, R.; Törnvall, U.; Gustafsson, L.; Börjesson, P. Industrial biotechnology for the production of bio-based chemicals—A cradle-to-grave perspective. Trends Biotechnol. 2007, 25, 119–124. [Google Scholar] [CrossRef] [PubMed]
- RJohn, P.; Nampoothiri, K.M.; Pandey, A. Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 524–534. [Google Scholar]
- Cui, X.; Zhao, X.; Liu, D. A novel route for the flexible preparation of hydrocarbon jet fuels from biomass-based platform chemicals: A case of using furfural and 2,3-butanediol as feedstocks. Green Chem. 2018, 20, 2018–2026. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Chen, K. Mechanisms and Clinical Application of Tetramethylpyrazine (An Interesting Natural Compound Isolated from Ligusticum Wallichii): Current Status and Perspective. Oxidative Med. Cell. Longev. 2016, 2016, 2124638. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, R.; Yang, S.; Phang, Y.; Zheng, C.; Zhang, H. The Protective Effects and Potential Mechanisms of Ligusticum chuanxiong: Focus on Anti-Inflammatory, Antioxidant, and Antiapoptotic Activities. Evid.-Based Complement. Altern. Med. 2020, 2020, 8205983. [Google Scholar] [CrossRef]
- Guo, M.; Liu, Y.; Shi, D. Cardiovascular Actions and Therapeutic Potential of Tetramethylpyrazine (Active Component Isolated from Rhizoma Chuanxiong): Roles and Mechanisms. BioMed Res. Int. 2016, 2016, 2430329. [Google Scholar] [CrossRef]
- Shao, H.; Zhao, L.; Chen, F.; Zeng, S.; Liu, S.; Li, J. Efficacy of Ligustrazine Injection as Adjunctive Therapy for Angina Pectoris: A Systematic Review and Meta-Analysis. Experiment 2015, 21, 3704–3715. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Zhang, Y. Characterization of Pyrazines in Some Chinese Liquors and Their Approximate Concentrations. J. Agric. Food Chem. 2007, 55, 9956–9962. [Google Scholar] [CrossRef]
- Xiao, Z.; Dai, S.; Niu, Y.; Yu, H.; Zhu, J.; Tian, H.; Gu, Y. Discrimination of Chinese Vinegars Based on Headspace Solid-Phase Microextraction-Gas Chromatography Mass Spectrometry of Volatile Compounds and Multivariate Analysis. J. Food Sci. 2011, 76, C1125–C1135. [Google Scholar] [CrossRef]
- Xiao, Z.; Hou, X.; Lyu, X.; Xi, L.; Zhao, J.-Y. Accelerated green process of tetramethylpyrazine production from glucose and diammonium phosphate. Biotechnol. Biofuels 2014, 7, 106. [Google Scholar] [CrossRef]
- Hao, F.; Wu, Q.; Xu, Y. Precursor Supply Strategy for Tetramethylpyrazine Production by Bacillus Subtilis on Solid-State Fermentation of Wheat Bran. Appl. Biochem. Biotechnol. 2013, 169, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kim, K.-S.; Shon, D.-H.; Chung, D.-K. Optimum conditions for the production of retramethylpyrazine dlavor compound by aerobic fed-batch culture of Lactococcus lactis subsup. lactis biovar. diacetylactis FC1. J. Microbiol. Biotechnol. 1994, 4, 327–332. [Google Scholar]
- Meng, W.; Ding, F.; Wang, R.-M.; Wang, T.-F. Enhanced Production of Tetramethylpyrazine in Bacillus licheniformis BL1 through aldC Over-expression and acetaldehyde Supplementation. Sci. Rep. 2020, 10, 3544. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, C.; Li, X.; Sun, B.; Eldin, A.A.; Jia, Y. A combinational optimization method for efficient synthesis of tetramethylpyrazine by the recombinant Escherichia coli. Biochem. Eng. J. 2018, 129, 33–43. [Google Scholar] [CrossRef]
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2022, 54, 107783. [Google Scholar] [CrossRef]
- Syu, M.J. Biological production of 2,3-butanediol. Appl. Microbiol. Biotechnol. 2001, 55, 10–18. [Google Scholar] [CrossRef]
- Celinska, E.; Grajek, W. Biotechnological production of 2,3-butanediol-Current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef]
- Yan, Y.; Lee, C.-C.; Liao, J.C. Enantioselective synthesis of pure (R,R)-2,3-butanediol in Escherichia coli with stereospecific secondary alcohol dehydrogenases. Org. Biomol. Chem. 2009, 7, 3914–3917. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Q.; Zhan, S.; Li, Y.; Lin, H.; Sun, S.; Sha, L.; Hu, K.; Guan, X.; Shen, Y. A new NAD(H)-dependent meso-2,3-butanediol dehydrogenase from an industrially potential strain Serratia marcescens H30. Appl. Microbiol. Biotechnol. 2014, 98, 1175–1184. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Zhang, L.; Ma, C.; Wang, A.; Tao, F.; Xu, P. Biocatalytic production of (2S,3S)-2,3-butanediol from diacetyl using whole cells of engineered Escherichia coli. Bioresour. Technol. 2012, 115, 111–116. [Google Scholar] [CrossRef]
- Soltys, K.A.; Batta, A.K.; Koneru, B. Successful Nonfreezing, Subzero Preservation of Rat Liver with 2,3-Butanediol and Type I Antifreeze Protein. J. Surg. Res. 2001, 96, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, L.; Xie, Y.; Hu, C.; Zhang, Y.; Li, L.; Wang, Y.; Ma, C.; Xu, P. Production of (3S)-acetoin from diacetyl by using stereoselective NADPH-dependent carbonyl reductase and glucose dehydrogenase. Bioresour. Technol. 2013, 137, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.J.; Xu, P. Acetoin metabolism in bacteria. Crit. Rev. Microbiol. 2007, 33, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.-J.; Huang, H.; Ouyang, P.-K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, M.; Liu, W.X.; Chen, T. Latest Advances of Microbial Production of 2,3-Butanediol. Prog. Chem. 2012, 24, 2268–2276. [Google Scholar]
- Meng, W.; Wang, R.; Xiao, D. Metabolic engineering of Bacillus subtilis to enhance the production of tetramethylpyrazine. Biotechnol. Lett. 2015, 37, 2475–2480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, X.; Zhang, J.; Zhao, C.; Liu, J.; Tian, Y.; Yang, L. Effect of deletion of 2,3-butanediol dehydrogenase gene (bdhA) on acetoin production of Bacillus subtilis. Prep. Biochem. Biotechnol. 2017, 47, 761–767. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Q.; Yu, M.; Wang, Y.; Xiong, B.; Zhang, Y.; Zheng, J.; Ying, X. Characterization of a stereospecific acetoin(diacetyl) reductase from Rhodococcus erythropolis WZ010 and its application for the synthesis of (2S,3S)-2,3-butanediol. Appl. Microbiol. Biotechnol. 2014, 98, 641–650. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ma, C.; Gao, C.; Tao, F.; Xu, P. Engineering of cofactor regeneration enhances (2S,3S)-2,3-butanediol production from diacetyl. Sci. Rep. 2013, 3, 2643. [Google Scholar] [CrossRef]
- Du, C.; Yan, H.; Zhang, Y.; Li, Y.; Cao, Z. Use of oxidoreduction potential as an indicator to regulate 1,3-propanediol fermentation by Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2006, 69, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Riondet, C.; Cachon, R.; Waché, Y.; Alcaraz, G.; Diviès, C. Extracellular oxidoreduction potential modifies carbon and electron flow in Escherichia coli. J. Bacteriol. 2000, 182, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Sakimoto, K.K.; Wong, A.B.; Yang, P. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 2016, 351, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, H.; Tian, Z.; Lu, D.; Yu, Y.; Cestellos-Blanco, S.; Sakimoto, K.K.; Yang, P. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production. Nat. Nanotechnol. 2018, 13, 900–905. [Google Scholar] [CrossRef]
- Guo, J.; Suástegui, M.; Sakimoto, K.K.; Moody, V.M.; Xiao, G.; Nocera, D.G.; Joshi, N.S. Light-driven fine chemical production in yeast biohybrids. Science 2018, 362, 813–816. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, C.; Chu, K.H.; Wu, D.; Yip, H.Y.; Ye, L.; Wong, P.K. Enhanced biological hydrogen production from Escherichia coli with surface precipitated cadmium sulfide nanoparticles. Adv. Energy Mater. 2017, 7, 1700611. [Google Scholar] [CrossRef]
- Song, J.; Lin, H.; Zhao, G.; Huang, X. Photocatalytic Material-Microorganism Hybrid System and Its Application—A Review. Micromachines 2022, 13, 861. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Mao, X.; Lao, Y.; Chen, F. Enhanced Photosynthesis of Carotenoids in Microalgae Driven by Light-Harvesting Gold Nanoparticles. ACS Sustain. Chem. Eng. 2020, 8, 7600–7608. [Google Scholar] [CrossRef]











| Plasmids and Primers | Description | Source |
|---|---|---|
| pBE980a | Kmr; P43 promoter; expressing vector | Laboratory stock |
| pBE980a-Plaps | pBE980a carries Plaps gene | This study |
| pBE980a-Plaps-bdhA | pBE980a carries Plaps, bdhA gene | This study |
| pBE980a(P43)-Plaps-bdhA | pBE980a carries Plaps, bdhA gene, deletes P43 gene | This study |
| pUC-sp | Kmr; carries Plaps gene | This study |
| bdhA-F | ggtacccggggatcctctagaATGAAGGCAGCAAGATGGCA | This study |
| bdhA-R | tggaattgtgctgaagctagcTTAGTTAGGTCTAACAAGGATTTTGACTT | This study |
| PBE980a-P-F | tcaagcttttgcctcgagctcGAGCTCTCAGGAGCATTTAACCTAA | This study |
| PBE980a-P-R | tgccttcattctagaggatccGGATCCCGTTCATGTCTCCTT | This study |
| PBE980a-bdhA-F | ggtacccggggatcctctagaATGAAGGCAGCAAGATGGCA | This study |
| PBE98a-bdhA-R | tggaattgtgctgaagctagcTTAGTTAGGTCTAACAAGGATTTTGACTT | This study |
| PUC-Plaps-F | caggtcgactctagaggatccCGTTCATGTCTCCTTTTTTATGTACTG | This study |
| PUC-Plaps-R | tcaagcttttgcctcgagctcTCAGGAGCATTTAACCTAAAAAAGC | This study |
| Fermentation Characterization | Strains | |||
|---|---|---|---|---|
| BS2 | BS-ppb11 | BS-43A | BS-LA26 | |
| Glucose (g/L) | 190 ± 1.5 | 191 ± 1.8 | 190 ± 2.0 | 187 ± 1.4 |
| Glucose consumption rate | 0.99 ± 0.01 | 1.14 ± 0.02 | 1.05 ± 0.02 | 1.06 ± 0.01 |
| 2,3-BD | 43.1 ± 0.9 | 58.8 ± 1.0 | 47.4 ± 1.1 | 33.0 ± 1.1 |
| 2,3-BD yield | 0.23 ± 0.02 | 0.35 ± 0.02 | 0.29 ± 0.01 | 0.19 ± 0.02 |
| Acetoin | 39.0 ± 1.1 | 53.3 ± 1.3 | 43.9 ± 1.2 | 52.8 ± 1.1 |
| Acetoin yield | 0.21 ± 0.02 | 0.32 ± 0.01 | 0.26 ± 0.03 | 0.31 ± 0.03 |
| Formic acid | 4.4 ± 0.12 | 2.9 ± 0.15 | 4.2 ± 0.2 | 4.5 ± 0.11 |
| Acetic acid | 7.7 ± 0.21 | 4.5 ± 0.16 | 5.5 ± 0.14 | 6.2 ± 0.16 |
| Lactic acid | 12.3 ± 0.23 | 6.3 ± 0.23 | 8.2 ± 0.25 | 8.8 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Lin, Y.; Song, J.; Li, H.; Gao, Y.; Lin, Y.; Huang, X.; Meng, W.; Qin, W. Engineered Bacillus subtilis for the Production of Tetramethylpyrazine,(R,R)-2,3-Butanediol and Acetoin. Fermentation 2023, 9, 488. https://doi.org/10.3390/fermentation9050488
Shi L, Lin Y, Song J, Li H, Gao Y, Lin Y, Huang X, Meng W, Qin W. Engineered Bacillus subtilis for the Production of Tetramethylpyrazine,(R,R)-2,3-Butanediol and Acetoin. Fermentation. 2023; 9(5):488. https://doi.org/10.3390/fermentation9050488
Chicago/Turabian StyleShi, Lin, Yuan Lin, Jiaao Song, Hongxing Li, Yinhao Gao, Yonghong Lin, Xiaowen Huang, Wu Meng, and Weishuai Qin. 2023. "Engineered Bacillus subtilis for the Production of Tetramethylpyrazine,(R,R)-2,3-Butanediol and Acetoin" Fermentation 9, no. 5: 488. https://doi.org/10.3390/fermentation9050488
APA StyleShi, L., Lin, Y., Song, J., Li, H., Gao, Y., Lin, Y., Huang, X., Meng, W., & Qin, W. (2023). Engineered Bacillus subtilis for the Production of Tetramethylpyrazine,(R,R)-2,3-Butanediol and Acetoin. Fermentation, 9(5), 488. https://doi.org/10.3390/fermentation9050488