Effect of Elevated Oxygen Concentration on the Yeast Yarrowia lipolytica for the Production of γ-Decalactones in Solid State Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solid Support, Biotransformation Medium, Strain and Inoculum Preparation
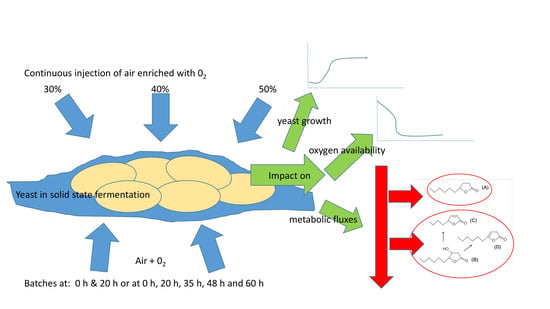
2.2. Solid State Fermentation Conditions
2.3. Injection System of Oxygen-Enriched Air
2.4. Gases Measurement and Biomass Estimation
2.5. Extraction and Quantification of Lactones
2.6. Modelling of Cells Growth, Oxygen Consumption and Lactone Productions
2.7. Statistical Analysis
3. Results
3.1. Growth of Y. lipolytica W29 Depends on the Oxygen Ratio
3.2. Oxygen Consumption of Y. lipolytica W29 Depends on the Oxygen Ratio
3.3. Production of Lactones Depends on the Oxygen Ratio
3.4. Production of Lactones Using Gas with an Oxygen Ratio of 30% Injected at Different Times
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Endrizzi, A.; Pagot, Y.; Le Clainche, A.; Nicaud, J.-M.; Belin, J.-M. Production of Lactones and Peroxisomal Beta-Oxidation in Yeasts. Crit. Rev. Biotechnol. 1996, 16, 301–329. [Google Scholar] [CrossRef] [PubMed]
- Waché, Y.; Aguedo, M.; Nicaud, J.-M.; Belin, J.-M. Catabolism of hydroxyacids and biotechnological production of lactones by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2003, 61, 393–404. [Google Scholar] [CrossRef]
- Karaalioğlu, O.; Yüceer, Y.K. Nonconventional yeasts to produce aroma compounds by using agri-food waste materials. FEMS Yeast Res. 2021, 21, foab063. [Google Scholar] [CrossRef]
- Małajowicz, J.; Kozłowska, M. Factors Affecting the Yield in Formation of Fat-Derived Fragrance Compounds by Yarrowia lipolytica Yeast. Appl. Sci. 2021, 11, 9843. [Google Scholar] [CrossRef]
- Gatfield, I.L.; Güntert, M.; Sommer, H.; Werkhoff, P. Some aspects of the microbiological production of flavor: Active lactones with particular reference to γ-decalactone. Chem. Mikrobiol. Technol. Lebensm. 1993, 15, 165–170. [Google Scholar]
- Waché, Y.; Aguedo, M.; Choquet, A.; Gatfield, I.L.; Nicaud, J.M.; Belin, J.M. Role of β-oxidation enzymes in γ-decalactone production by the yeast Yarrowia lipolytica. Appl. Environ. Microbiol. 2001, 67, 5700–5704. [Google Scholar] [CrossRef] [PubMed]
- Try, S.; De-Coninck, J.; Voilley, A.; Chunhieng, T.; Waché, Y. Solid state fermentation for the production of γ-decalactones by Yarrowia lipolytica. Process. Biochem. 2018, 64, 9–15. [Google Scholar] [CrossRef]
- García, E.E.; Aguedo, M.; Gomes, N.; Choquet, A.; Belo, I.; Teixeira, J.A.; Belin, J.-M.; Waché, Y. Production of 3-hydroxy-γ-decalactone, the precursor of two decenolides with flavouring properties, by the yeast Yarrowia lipolytica. J. Molec. Catal. B 2009, 57, 22–26. [Google Scholar] [CrossRef]
- Waché, Y. Production of dicarboxylic acids and flagrances by Yarrowia lipolytica. In Yarrowia lipolytica; Springer: Berlin/Heidelberg, Germany, 2013; pp. 151–170. [Google Scholar]
- Fickers, P.; Benetti, P.-H.; Waché, Y.; Marty, A.; Mauersberger, S.; Smit, M.S.; Nicaud, J.-M. Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res. 2005, 5, 527–543. [Google Scholar] [CrossRef]
- Wang, H.; Le Clainche, A.; Le Dall, M.T.; Wache, Y.; Pagot, Y.; Belin, J.M.; Gaillardin, C.; Nicaud, J.M. Cloning and characterization of the peroxisomal acyl CoA oxidase ACO3 gene from the alkane-utilizing yeast Yarrowia lipolytica. Yeast 1998, 14, 1373–1386. [Google Scholar] [CrossRef]
- Pagot, Y.; Le Clainche, A.; Nicaud, J.-M.; Wache, Y.; Belin, J.-M. Peroxisomal β-oxidation activities and γ-decalactone production by the yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 1998, 49, 295–300. [Google Scholar] [CrossRef]
- Waché, Y.; Laroche, C.; Bergmark, K.; Møller-Andersen, C.; Aguedo, M.; Le Dall, M.-T.; Wang, H.; Nicaud, J.-M.; Belin, J.-M. Involvement of acyl Coenzyme A oxidase isozymes in biotransformation of methyl ricinoleate into γ-decalactone by Yarrowia lipolytica. Appl. Environ. Microbiol. 2000, 66, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Groguenin, A.; Waché, Y.; Garcia, E.E.; Aguedo, M.; Husson, F.; LeDall, M.-T.; Nicaud, J.-M.; Belin, J.-M. Genetic engineering of the β-oxidation pathway in the yeast Yarrowia lipolytica to increase the production of aroma compounds. J. Mol. Catal. B 2004, 28, 75–79. [Google Scholar] [CrossRef]
- Escamilla-García, E.; Nicaud, J.M.; Belin, J.M.; Waché, Y. Effect of acyl-CoA oxidase activity on the accumulation of γ-decalactone by the yeast Yarrowia lipolytica: A factorial approach. Biotechnol. J. 2007, 2, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-García, E.; Belin, J.M.; Waché, Y. Use of a Doehlert factorial design to investigate the effects of pH and aeration on the accumulation of lactones by Yarrowia lipolytica. J. Appl. Microbiol. 2007, 103, 1508–1515. [Google Scholar] [CrossRef]
- Escamilla-García, E.; O’riordan, S.; Gomes, N.; Aguedo, M.; Belo, I.; Teixeira, J.; Belin, J.-M.; Waché, Y. An air-lift biofilm reactor for the production of γ-decalactones by Yarrowia lipolytica. Process. Biochem. 2014, 49, 1377–1382. [Google Scholar] [CrossRef]
- Braga, A.; Belo, I. Production of γ-decalactone by Yarrowia lipolytica: Insights into experimental conditions and operating mode optimization. J. Chem. Technol. Biotechnol. 2015, 90, 559–565. [Google Scholar] [CrossRef]
- Waché, Y.; Dijon, A. Microbial production of food flavours. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; McNeil, B., Archer, D., Giavasis, I., Harvey, L., Eds.; Woodhead: Cambridge, UK, 2013; pp. 175–193. [Google Scholar]
- Braga, A.; Mesquita, D.P.; Amaral, A.L.; Ferreira, E.C.; Belo, I. Aroma production by Yarrowia lipolytica in airlift and stirred tank bioreactors: Differences in yeast metabolism and morphology. Biochem. Eng. J. 2015, 93, 55–62. [Google Scholar] [CrossRef]
- ALMualad, W.N.A.; Bouchedja, D.N.; Selmania, A.; Maadadi, R.; Ikhlef, A.; Kabouche, Z.; Elmechta, L.; Boudjellal, A. Yeast Yarrowia lipolytica as a biofactory for the production of lactone-type aroma gamma-decalactone using castor oil as substrate. Chem. Pap. 2022, 76, 7715–7728. [Google Scholar] [CrossRef]
- Baez, A.; Shiloach, J. Effect of elevated oxygen concentration on bacteria, yeasts, and cells propagated for production of biological compounds. Microb. Cell Factories 2014, 13, 181. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Mimi Sakinah, A.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef]
- Clark, D.S.; Blanch, H.W. Biochemical Engineering; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Garcia-Ochoa, F.; Gomez, E.; Santos, V.E.; Merchuk, J.C. Oxygen uptake rate in microbial processes: An overview. Biochem. Eng. J. 2010, 49, 289–307. [Google Scholar] [CrossRef]
- Noorman, H.J. Masse transfer. In Basic Biotechnology, 2nd ed.; Ratledge, C., Kristiansen, B., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 173–186. [Google Scholar]
- Moo-Young, M.; Blanch, H.W. Transport phenomena in microbial systems. In Basic Biotechnology; Bu’Lock, J.D., Kristiansen, B., Eds.; Academic Press: San Diego, CA, USA, 1987; pp. 133–168. [Google Scholar]
- Bailey, J.E.; Ollis, D.F. Transport phenomena in microbial systems. In Biochemical Engineering Fundamentals; Bailey, J.E., Ollis, D.F., Eds.; McGraw-Hill: New York, NY, USA, 1977; pp. 411–473. [Google Scholar]
- Korshunov, S.; Imlay, J.A. Detection and quantification of superoxide formed within the periplasm of Escherichia coli. J. Bacteriol. 2006, 188, 6326–6334. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Cellular defenses against superoxide and hydrogen peroxide. Ann. Rev. Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef]
- Messner, K.R.; Imlay, J.A. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J. Biol. Chem. 1999, 274, 10119–10128. [Google Scholar] [CrossRef]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef]
- Harley, J.B.; Santangelo, G.M.; Rasmussen, H.; Goldfine, H. Dependence of Escherichia coli hyperbaric oxygen toxicity on the lipid acyl chain composition. J. Bacteriol. 1978, 134, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Gille, J.J.P.; Wortelboer, H.M.; Joenje, H. Effect of normobaric hyperoxia on antioxidant defenses of HeLa and CHO cells. Free. Radic. Biol. Med. 1988, 4, 85–91. [Google Scholar] [CrossRef]
- Lin, A.A.; Miller, W.M. Modulation of glutathione level in CHO cells. Ann. N. Y. Acad. Sci. 1992, 665, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Scatena, R.; Messana, I.; Martorana, G.E.; Gozzo, M.L.; Lippa, S.; Maccaglia, A.; Bottoni, P.; Vincenzoni, F.; Nocca, G.; Castagnola, M.; et al. Mitochondrial damage and metabolic compensatory mechanisms induced by hyperoxia in the U-937 cell line. BMB Rep. 2004, 37, 454–459. [Google Scholar] [CrossRef]
- Gatfield, I.L. Biotechnological production of natural flavor materials. In Flavor Chemistry, Thirty Years of Progress; Teranishi, R., Wick, E.L., Hornstein, I., Eds.; Kluwer Academic, Plenum Publishers: New York, NY, USA, 1999; pp. 211–227. [Google Scholar]
- Okui, S.; Uchiyama, M.; Mizugaki, M. Metabolism of hydroxy fatty acids. J. Biochem. 1963, 54, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Feron, G.; Dufosse, L.; Pierard, E.V.A.; Bonnarme, P.; Quere, J.L.; Spinnler, H. Production, identification, and toxicity of γ-decalactone and 4-hydroxydecanoic acid from Sporidiobolus spp. Appl. Environ. Microbiol. 1996, 62, 2826–2831. [Google Scholar] [CrossRef] [PubMed]
- Waché, Y.; Husson, F.; Feron, G.; Belin, J.-M. Yeast as an efficient biocatalyst for the production of lipid-derived flavours and fragrances. Antonie Van Leeuwenhoek 2006, 89, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Endrizzi-Joran, A. Biotransformation de Ricinoleate de Méthyl en γ-décalactone par des Levures. Ph.D. Thesis, Université de Bourgogne, Dijon, France, 1994. [Google Scholar]
- Małajowicz, J.; Górska, A.; Bryś, J.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M. Attempt to Develop an Effective Method for the Separation of Gamma-Decalactone from Biotransformation Medium. Appl. Sci. 2022, 12, 2084. [Google Scholar] [CrossRef]









| Parameters | Experimental Conditions of Oxygen-Enriched Air * | |||
|---|---|---|---|---|
| 20% | 30% | 40% | 50% | |
| µmax (h−1) | 0.08 a | 0.14 b | 0.09 a | 0.08 a |
| Xmax (108 cells g−1 of dried weight) | 4.38 a | 5.50 b | 6.70 c | 6.91 c |
| Parameters | Unite | Experimental Conditions of Oxygen-Enriched Air ** | |||
|---|---|---|---|---|---|
| 20% | 30% | 40% | 50% | ||
| qO2max | mg (108 cells)−1 h−1 * | 5.15 | 9.57 | 7.60 | 6.18 |
| PO2max | mg L−1 | 275 | 401 | 561 | 676 |
| Parameters | Unite | Experimental Conditions of Oxygen-Enriched Air ** | |||
|---|---|---|---|---|---|
| 20% | 30% | 40% | 50% | ||
| qGDLmax | mg (108 cells)−1 h−1 * | 1.68 ab | 1.93 a | 1.03 bc | 0.74 c |
| q2DECmax | 2.07 a | 6.45 | 1.73 a | 1.61 a | |
| q3DECmax | 0.83 a | 1.06 a | 0.45 a | 0.45 a | |
| q3OHmax | 10.32 a | 29.91 | 12.52 a | 11.07 a | |
| PGDLmax | mg L−1 | 147 | 289 a | 227 b | 253 ab |
| P2DECmax | 121 a | 198 | 149 a | 159 a | |
| P3DECmax | 48 a | 47 a | 53 a | 50 a | |
| P3OHmax | 714 | 1197 a | 1497 ab | 1740 b | |
| nGDL | mole lactone per mole oxygen consumed | 0.061 | 0.038 | 0.024 a | 0.023 a |
| n2DEC | 0.077 | 0.128 | 0.043 a | 0.050 a | |
| n3DEC | 0.031 a | 0.021 ab | 0.011 b | 0.014 b | |
| n3OH | 0.345 a | 0.538 | 0.283 a | 0.308 a | |
| nTotal lactones | 0.513 a | 0.725 | 0.362 b | 0.394 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Try, S.; Voilley, A.; Chunhieng, T.; De-Coninck, J.; Waché, Y. Effect of Elevated Oxygen Concentration on the Yeast Yarrowia lipolytica for the Production of γ-Decalactones in Solid State Fermentation. Fermentation 2023, 9, 532. https://doi.org/10.3390/fermentation9060532
Try S, Voilley A, Chunhieng T, De-Coninck J, Waché Y. Effect of Elevated Oxygen Concentration on the Yeast Yarrowia lipolytica for the Production of γ-Decalactones in Solid State Fermentation. Fermentation. 2023; 9(6):532. https://doi.org/10.3390/fermentation9060532
Chicago/Turabian StyleTry, Sophal, Andrée Voilley, Thavarith Chunhieng, Joëlle De-Coninck, and Yves Waché. 2023. "Effect of Elevated Oxygen Concentration on the Yeast Yarrowia lipolytica for the Production of γ-Decalactones in Solid State Fermentation" Fermentation 9, no. 6: 532. https://doi.org/10.3390/fermentation9060532
APA StyleTry, S., Voilley, A., Chunhieng, T., De-Coninck, J., & Waché, Y. (2023). Effect of Elevated Oxygen Concentration on the Yeast Yarrowia lipolytica for the Production of γ-Decalactones in Solid State Fermentation. Fermentation, 9(6), 532. https://doi.org/10.3390/fermentation9060532