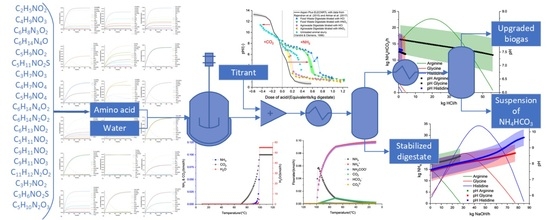
Modelling of Amino Acid Fermentations and Stabilization of Anaerobic Digestates by Extracting Ammonium Bicarbonate
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Preliminary Validation of the Aspen Plus® v12 ELECNRTL Methodology
3.2. Optimization of the Conditions of the Flash Distillation to Produce NH4HCO3
3.3. Anaerobic Fermentation of Amino Acids
3.4. Suitable Conditions for NH4HCO3-Stabilization of Anaerobic Digestate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A
| Component Name | Alias | CAS Number | mol/L |
|---|---|---|---|
| WATER | H2O | 7732-18-5 | 45.69365 |
| CARBON-DIOXIDE | CO2 | 124-38-9 | 0.013835 |
| AMMONIA | H3N | 7664-41-7 | 0.100484 |
| HYDROGEN-SULFIDE | H2S | 7783-06-4 | 0.001921 |
| ACETIC-ACID | C2H4O2-1 | 64-19-7 | 0.117655 |
| GLYCEROL | C3H8O3 | 56-81-5 | 0.000788 |
| OLEIC-ACID | C18H34O2 | 112-80-1 | 0.001519 |
| DEXTROSE | C6H12O6 | 50-99-7 | 0.119788 |
| PROPIONIC-ACID | C3H6O2-1 | 79-09-4 | 0.000969 |
| ETHYL-CYANOACETATE | C5H7NO2 | 105-56-6 | 4.19E-05 |
| DL-ALANINE | C3H7NO2-N9 | 302-72-7 | 0.000452 |
| ARGININE | C6H14N4O2-N2 | 7004-12-8 | 0.000433 |
| DL-ASPARTIC-ACID | C4H7NO4-N4 | 617-45-8 | 0.000462 |
| L-CYSTEINE | C3H7NO2S-N1 | 52-90-4 | 0.000645 |
| DL-GLUTAMIC-ACID | C5H9NO4-N5 | 617-65-2 | 0.000712 |
| GLYCINE | C2H5NO2-D1 | 56-40-6 | 0.002407 |
| L-ISOLEUCINE | C6H13NO2-N3 | 73-32-5 | 0.000448 |
| L-LEUCINE | C6H13NO2-N2 | 61-90-5 | 0.000674 |
| L-PHENYLALANINE | C9H11NO2 | 63-91-2 | 0.000347 |
| DL-PROLINE | C5H9NO2-N9 | 609-36-9 | 0.001069 |
| DL-SERINE | C3H7NO3-N5 | 302-84-1 | 0.001656 |
| C4H9NO3-N5 | C4H9NO3-N5 | 72-19-5 | 0.000452 |
| C5H11NO2-N17 | C5H11NO2-N17 | 516-06-3 | 0.000712 |
| GLUTARIC-ACID | C5H8O4 | 110-94-1 | 0.037695 |
| HYDROGEN | H2 | 1333-74-0 | 1.55E-06 |
| METHANE | CH4 | 74-82-8 | 0.000238 |
| MALTOSE | C12H22O11-N2 | 69-79-4 | 0.050679 |
| TRIOLEIN | C57H104O6 | 122-32-7 | 5.62E-05 |
| TRIPALMITIN | C51H98O6 | 555-44-2 | 6.18E-05 |
| 1-HEXADECANOL | C16H34O | 36653-82-4 | 6.86E-08 |
| 2-OLEODIPALMITIN | C53H100O6-N1 | 2190-25-2 | 6.18E-05 |
| TRILINOLENIN | C57H92O6 | 14465-68-0 | 8.4E-05 |
| BETA-D-XYLOPYRANOSE | C5H10O5-D3 | 2460-44-8 | 0.009424 |
| LINOLEIC-ACID | C18H32O2 | 60-33-3 | 0.000756 |
| ETHANOL | C2H6O-2 | 64-17-5 | 0.13388 |
| SODIUM-BICARBONATE | NAHCO3 | 144-55-8 | 0.044112 |
| CALCIUM-CHLORIDE | CACL2 | 10043-52-4 | 0.002876 |
| SODIUM-CHLORIDE | NACL | 7647-14-5 | 0.003601 |
| POTASSIUM-BICARBONATE | KHCO3 | 298-14-6 | 0.007789 |



References
- da Costa Gomez, C. Biogas as an energy option: An overview. In The Biogas Handbook: Science, Production and Applications, 1st ed.; Wellinger, A., Murphy, J.D., Baxter, D., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2013; pp. 1–16. [Google Scholar]
- Patel, S.K.S.; Das, D.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Lee, J.-K. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew. Sustain. Energy Rev. 2021, 150, 111491. [Google Scholar] [CrossRef]
- Seadi, T.A.; Lukehurst, C. Quality Management of Digestate from Biogas Plants Used as Fertiliser; IEA Bioenergy, 2012; p. 40. Available online: http://www.iea-biogas.net/files/daten-redaktion/download/publi-task37/digestate_quality_web_new.pdf (accessed on 29 July 2023).
- Lukehurst, C.; Frost, P.; Seadi, T.A. Utilisation of Digestate from Biogas Plants as Biofertiliser; IEA Bioenergy, 2010; Available online: https://task37.ieabioenergy.com/wp-content/uploads/sites/32/2022/02/Digestate_Brochure_Revised_12-2010.pdf (accessed on 29 July 2023).
- Astals, S.; Martínez-Martorell, M.; Huete-Hernández, S.; Aguilar-Pozo, V.B.; Dosta, J.; Chimenos, J.M. Nitrogen recovery from pig slurry by struvite precipitation using a low-cost magnesium oxide. Sci. Total Environ. 2021, 768, 144284. [Google Scholar] [CrossRef] [PubMed]
- Drosg, B.; Fuchs, W.; Al Seadi, T.; Madsen, M.; Linke, B. Nutrient Recovery by Biogas Digestate Processing. Available online: https://task37.ieabioenergy.com/wp-content/uploads/sites/32/2022/02/NUTRIENT_RECOVERY_RZ_web2.pdf (accessed on 14 December 2021).
- Fuchs, W.; Drosg, B. Assessment of the state of the art of technologies for the processing of digestate residue from anaerobic digesters. Water Sci. Technol. 2013, 67, 1984–1993. [Google Scholar] [CrossRef]
- Moller, K.; Muller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2015, 12, 242–257. [Google Scholar] [CrossRef]
- Burke, D.A. Removal of Ammonia from Fermentation Effluent and Sequestration as Ammonium Bicarbonate and/or Carbonate. 12. 2010. Available online: https://patents.google.com/patent/US7811455B2/en (accessed on 29 July 2023).
- Burke, D.A. Reclaiming Ammonia from Anaerobic Digestate As A Profitable Product. Proc. Water Environ. Fed. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Bassani, D.; Orentlicher, M.; Simon, M.M.; Pagano, S. Process to Recover Ammonium Bicarbonate from Wastewater. 2019. Available online: https://patents.google.com/patent/US10106447B2/en (accessed on 29 July 2023).
- Jamaludin, Z.; Rollings-Scattergood, S.; Lutes, K.; Vaneeckhaute, C. Evaluation of sustainable scrubbing agents for ammonia recovery from anaerobic digestate. Bioresour. Technol. 2018, 270, 596–602. [Google Scholar] [CrossRef]
- Drapanauskaite, D.; Handler, R.M.; Fox, N.; Baltrusaitis, J. Transformation of Liquid Digestate from the Solid-Separated Biogas Digestion Reactor Effluent into a Solid NH4HCO3 Fertilizer: Sustainable Process Engineering and Life Cycle Assessment. ACS Sustain. Chem. Eng. 2021, 9, 580–588. [Google Scholar] [CrossRef]
- Budzianowski, W.M. Benefits of biogas upgrading to biomethane by high-pressure reactive solvent scrubbing. Biofuels Bioprod. Biorefin. 2012, 6, 12–20. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Walker, M.; Banks, C.; Heaven, S.; Frederickson, J. Residual Biogas Potential Test for Digestates; WRAP: Banbury, UK, 2010; Available online: https://www.ktbl.de/fileadmin/user_upload/Allgemeines/Download/Ringversuch-Biogas/Residual-Biogas-Potential.pdf (accessed on 29 July 2023).
- Saveyn, H.; Eder, P. End-of-Waste Criteria for Biodegradable Waste Subjected to Biological Treatment (Compost & Digestate): Technical Proposals. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC87124 (accessed on 14 December 2021).
- WRAP. BSI PAS 110:2014 Specification for Whole Digestate, Separated Liquor and Separated Fibre Derived from the Anaerobic Digestion of Source-Segregated Biodegradable Materials. Available online: https://wrap.org.uk/sites/default/files/2021-03/PAS110_2014.pdf (accessed on 7 January 2022).
- Rajendran, K.; Kankanala, H.R.; Lundin, M.; Taherzadeh, M.J. A novel process simulation model (PSM) for anaerobic digestion using Aspen Plus. Bioresour. Technol. 2014, 168, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Centorcelli, J.C.; Drapanauskaite, D.; Handler, R.M.; Baltrusaitis, J. Solar Steam Generation Integration into the Ammonium Bicarbonate Recovery from Liquid Biomass Digestate: Process Modeling and Life Cycle Assessment. ACS Sustain. Chem. Eng. 2021, 9, 15278–15286. [Google Scholar] [CrossRef]
- Centorcelli, J.C.; Luyben, W.L.; Romero, C.E.; Baltrusaitis, J. Dynamic Control of Liquid Biomass Digestate Distillation Combined with an Integrated Solar Concentrator Cycle for Sustainable Nitrogen Fertilizer Production. ACS Sustain. Chem. Eng. 2022, 10, 7409–7417. [Google Scholar] [CrossRef]
- Akhiar, A.; Battimelli, A.; Torrijos, M.; Carrere, H. Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag. 2017, 59, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Moure Abelenda, A.; Semple, K.T.; Lag-brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Strategies for the production of a stable blended fertilizer of anaerobic digestates and wood ashes. Nat. Based Solut. 2022, 2, 10014. [Google Scholar] [CrossRef]
- Government, U.K. Annex 3: FETF 2023 Productivity and Slurry Eligible Items. 2023. Available online: https://www.gov.uk/government/publications/farming-equipment-and-technology-fund-fetf-2023/annex-3-fetf-2023-productivity-and-slurry-eligible-items (accessed on 29 July 2023).
- García-Ochoa, F.; Santos, V.E.; Naval, L.; Guardiola, E.; López, B. Kinetic model for anaerobic digestion of livestock manure. Enzym. Microb. Technol. 1999, 25, 55–60. [Google Scholar] [CrossRef]
- Canu, P.; Pagin, M. Biogas upgrading by 2-steps methanation of its CO2—Thermodynamics analysis. J. CO2 Util. 2022, 63, 102123. [Google Scholar] [CrossRef]
- Darde, V. CO2 Capture Using Aqueous Ammonia. 2011. Available online: https://www.cere.dtu.dk/-/media/centre/cere/publications/phd-thesis/2011/victor_darde_phd.pdf?la=da&hash=8A67D4D04AFF063B772D6A90CC6D88B9BEBAEABD (accessed on 29 July 2023).
- Darde, V.; van Well, W.J.M.; Stenby, E.H.; Thomsen, K. Modeling of Carbon Dioxide Absorption by Aqueous Ammonia Solutions Using the Extended UNIQUAC Model. Ind. Eng. Chem. Res. 2010, 49, 12663–12674. [Google Scholar] [CrossRef]
- Mullin, J.W. Crystallization, 4th ed.; Butterworth-Heinemann: Oxford, UK; Boston, MA, USA, 2001; Table A.4. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 91st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Brondi, M.; Eisa, M.; Bortoletto-Santos, R.; Drapanauskaite, D.; Reddington, T.; Williams, C.; Ribeiro, C.; Baltrusaitis, J. Recovering, Stabilizing, and Reusing Nitrogen and Carbon from Nutrient-Containing Liquid Waste as Ammonium Carbonate Fertilizer. Agriculture 2023, 13, 909. [Google Scholar] [CrossRef]
- Vandré, R.; Clemens, J. Studies on the relationship between slurry pH, volatilization processes and the influence of acidifying additives. Nutr. Cycl. Agroecosyst. 1996, 47, 157–165. [Google Scholar] [CrossRef]
- Mu, Z.X.; He, C.S.; Jiang, J.K.; Zhang, J.; Yang, H.Y.; Mu, Y. A modified two-point titration method for the determination of volatile fatty acids in anaerobic systems. Chemosphere 2018, 204, 251–256. [Google Scholar] [CrossRef]
- Green, D.W.; Perry, R.H. Perry’s Chemical Engineers’ Handbook, 8th ed.; McGraw-Hill: New York, NY, USA; London, UK, 2008; pp. 140–145. [Google Scholar]
- Limoli, A.; Langone, M.; Andreottola, G. Ammonia removal from raw manure digestate by means of a turbulent mixing stripping process. J. Environ. Manag. 2016, 176, 1–10. [Google Scholar] [CrossRef]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Anaerobic co-digestion of pig manure and crude glycerol at mesophilic conditions: Biogas and digestate. Bioresour. Technol. 2012, 110, 63–70. [Google Scholar] [CrossRef]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Thermophilic co-digestion of pig manure and crude glycerol: Process performance and digestate stability. J. Biotechnol. 2013, 166, 97–104. [Google Scholar] [CrossRef]
- European Sustainable Phosphorus Platform. 1st White Ammonia Research Meeting (WARM)—7th June 2023. 2023. Available online: https://www.phosphorusplatform.eu/activities/conference/n-recovery (accessed on 29 July 2023).
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry—A review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Ukwuani, A.T.; Tao, W. Developing a vacuum thermal stripping—Acid absorption process for ammonia recovery from anaerobic digester effluent. Water Res. 2016, 106, 108–115. [Google Scholar] [CrossRef]
- Martín-Hernández, E.; Sampat, A.M.; Zavala, V.M.; Martín, M. Optimal integrated facility for waste processing. Chem. Eng. Res. Des. 2018, 131, 160–182. [Google Scholar] [CrossRef]
- Morey, L.; Fernández, B.; Tey, L.; Biel, C.; Robles-Aguilar, A.; Meers, E.; Soler, J.; Porta, R.; Cots, M.; Riau, V. Acidification and solar drying of manure-based digestate to produce improved fertilizing products. J. Environ. Manag. 2023, 336, 117664. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Semple, K.T.; Aggidis, G.; Aiouache, F. Dataset on the solid-liquid separation of anaerobic digestate by means of wood ash-based treatment. Data Brief 2022, 44, 108536. [Google Scholar] [CrossRef]
- Meixner, K.; Fuchs, W.; Valkova, T.; Svardal, K.; Loderer, C.; Neureiter, M.; Bochmann, G.; Drosg, B. Effect of precipitating agents on centrifugation and ultrafiltration performance of thin stillage digestate. Sep. Purif. Technol. 2015, 145, 154–160. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Saché, E.L.; Hurd Price, C.A.; Reina, T.R.; Navarrete, B. From biogas upgrading to CO2 utilization and waste recycling: A novel circular economy approach. J. CO2 Util. 2021, 47, 101496. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Ali, A.; Semple, K.T.; Aiouache, F. Aspen Plus® Process Simulation Model of the Biomass Ash-Based Treatment of Anaerobic Digestate for Production of Fertilizer and Upgradation of Biogas. Energies 2023, 16, 3039. [Google Scholar] [CrossRef]
- Aspen Technology. Rate-Based Model of the CO2 Capture Process by NH3 Using Aspen Plus Aspen Plus; Aspen Technology: Bedford, MA, USA, 2008. [Google Scholar]
- Moure Abelenda, A. Aspen Plus Models of the Flash Distillation Process for Stabilization of Anaerobic Digestate and Synthesis of Ammonium Bicarbonate. 2023. Available online: https://www.zenodo.org/record/7738947 (accessed on 29 July 2023).






| A | B | C | D | |
|---|---|---|---|---|
| 231.465424 | 12092.099609 | 36.781601 | 0 | |
| 216.050446 | 12431.700195 | 35.481899 | 0 | |
| 1.256563 | 3335.699951 | 1.4971 | −0.037057 | |
| 4.583437 | 2900 | 0 | 0 | |
| 554.818115 | 22442.529297 | 89.006416 | 0.064732 | |
| 4.289233 | 0 | 0 | 0 |
| k/(d−1) | E/(cal/mol) | |
|---|---|---|
| 4.32·1013 | 13.249 | |
| 2.38·1017 | 29.451 | |
| 1.35·1011 | 11.585 | |
| 2.14·1021 | 17.203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moure Abelenda, A.; Aggidis, G.; Aiouache, F. Modelling of Amino Acid Fermentations and Stabilization of Anaerobic Digestates by Extracting Ammonium Bicarbonate. Fermentation 2023, 9, 750. https://doi.org/10.3390/fermentation9080750
Moure Abelenda A, Aggidis G, Aiouache F. Modelling of Amino Acid Fermentations and Stabilization of Anaerobic Digestates by Extracting Ammonium Bicarbonate. Fermentation. 2023; 9(8):750. https://doi.org/10.3390/fermentation9080750
Chicago/Turabian StyleMoure Abelenda, Alejandro, George Aggidis, and Farid Aiouache. 2023. "Modelling of Amino Acid Fermentations and Stabilization of Anaerobic Digestates by Extracting Ammonium Bicarbonate" Fermentation 9, no. 8: 750. https://doi.org/10.3390/fermentation9080750
APA StyleMoure Abelenda, A., Aggidis, G., & Aiouache, F. (2023). Modelling of Amino Acid Fermentations and Stabilization of Anaerobic Digestates by Extracting Ammonium Bicarbonate. Fermentation, 9(8), 750. https://doi.org/10.3390/fermentation9080750