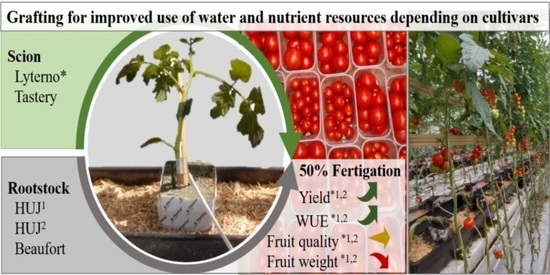
Novel S. pennellii × S. lycopersicum Hybrid Rootstocks for Tomato Production with Reduced Water and Nutrient Supply
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. Determination of Total Yield per Plant, Single Fruit Weight and Water-Use Efficiency
2.3. Determination of Total Soluble Solids
2.4. Determination of Flavonoid and Lycopene Content
2.5. Data Analysis
3. Results
4. Discussion
4.1. Impact of Different Rootstocks on Scion Performance
4.2. Effects of Rootstocks on Water-Use Efficiency during Cultivation Cycle
4.3. Rootstock Influence on Fruit Quality Parameters
4.4. Future Perspective of Renewable Substrate for Hydroponics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Rodríguez, E.; Leyva, R.; Constán-Aguilar, C.; Romero, L.; Ruiz, J.M. Grafting under Water Stress in Tomato Cherry: Improving the Fruit Yield and Quality. Ann. Appl. Biol. 2012, 161, 302–312. [Google Scholar] [CrossRef]
- Ullah, H.; Santiago-Arenas, R.; Ferdous, Z.; Attia, A.; Datta, A. Chapter Two—Improving Water Use Efficiency, Nitrogen Use Efficiency, and Radiation Use Efficiency in Field Crops under Drought Stress: A Review. Adv. Agron. 2019, 156, 109–157. [Google Scholar] [CrossRef]
- Cherry, C.; Scott, C.; Barrett, J.; Pidgeon, N. Public Acceptance of Resource-Efficiency Strategies to Mitigate Climate Change. Nat. Clim. Chang. 2018, 8, 1007–1012. [Google Scholar] [CrossRef]
- Cuartero, J.; Bolarin, M.C.; Asins, M.J.; Moreno, V. Increasing Salt Tolerance in the Tomato. J. Exp. Bot. 2006, 57, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and Future Use of Wild Relatives in Crop Breeding. Crop. Sci. 2017, 57, 1070–1082. [Google Scholar] [CrossRef]
- Bolger, A.; Scossa, F.; Bolger, M.E.; Lanz, C.; Maumus, F.; Tohge, T.; Quesneville, H.; Alseekh, S.; Sørensen, I.; Lichtenstein, G.; et al. The Genome of the Stress-Tolerant Wild Tomato Species Solanum Pennellii. Nat. Genet. 2014, 46, 1034–1038. [Google Scholar] [CrossRef]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De Novo Domestication of Wild Tomato Using Genome Editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef] [Green Version]
- Zimny, T.; Sowa, S.; Tyczewska, A.; Twardowski, T. Certain New Plant Breeding Techniques and Their Marketability in the Context of EU GMO Legislation—Recent Developments. New Biotechnol. 2019, 51, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. CRISPR Plants Now Subject to Tough GM Laws in European Union. Nature 2018, 560, 16. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Colla, G.; Zrenner, R.; Schwarz, D. Vegetable Grafting: The Implications of a Growing Agronomic Imperative for Vegetable Fruit Quality and Nutritive Value. Front. Plant Sci. 2017, 8, 741. [Google Scholar] [CrossRef]
- Rouphael, Y.; Schwarz, D.; Krumbein, A.; Colla, G. Impact of Grafting on Product Quality of Fruit Vegetables. Sci. Hortic. 2010, 127, 172–179. [Google Scholar] [CrossRef]
- Alan, O.; Ozdemir, N.; Gunen, Y. Effect of Grafting on Watermelon Plant Growth, Yield and Quality. J. Agron. 2007, 6, 362–365. [Google Scholar] [CrossRef] [Green Version]
- López-Pérez, J.-A.; Le Strange, M.; Kaloshian, I.; Ploeg, A.T. Differential Response of Mi Gene-Resistant Tomato Rootstocks to Root-Knot Nematodes (Meloidogyne Incognita). Crop. Prot. 2006, 25, 382–388. [Google Scholar] [CrossRef]
- Rivard, C.L.; O’Connell, S.; Peet, M.M.; Welker, R.M.; Louws, F.J. Grafting Tomato to Manage Bacterial Wilt Caused by Ralstonia Solanacearum in the Southeastern United States. Plant Dis. 2012, 96, 973–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estan, M.T.; Martinez-Rodriguez, M.M.; Perez-Alfocea, F.; Flowers, T.J.; Bolarin, M.C. Grafting Raises the Salt Tolerance of Tomato through Limiting the Transport of Sodium and Chloride to the Shoot. J. Exp. Bot. 2005, 56, 703–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savvas, D.; Colla, G.; Rouphael, Y.; Schwarz, D. Amelioration of Heavy Metal and Nutrient Stress in Fruit Vegetables by Grafting. Sci. Hortic. 2010, 127, 156–161. [Google Scholar] [CrossRef]
- Flores, F.B.; Sanchez-Bel, P.; Estañ, M.T.; Martinez-Rodriguez, M.M.; Moyano, E.; Morales, B.; Campos, J.F.; Garcia-Abellán, J.O.; Egea, M.I.; Fernández-Garcia, N.; et al. The Effectiveness of Grafting to Improve Tomato Fruit Quality. Sci. Hortic. 2010, 125, 211–217. [Google Scholar] [CrossRef]
- Statistisches-Bundesamt. Erntemenge von Tomaten in Deutschland in Den. Jahren 2001 Bis 2019 (in 1.000 Tonnen); Statistisches Bundesamt Destatis: Wiesbaden, Germany, 2020. [Google Scholar]
- Altunlu, H.; Gul, A. Increasing Drought Tolerance of Tomato Plants by Grafting. Acta Hortic. 2012, 183–190. [Google Scholar] [CrossRef]
- Öztekin, G.B.; Tuzel, Y. Salinity Response of Some Tomato Rootstocks at Seedling Stage. Afr. J. Agric. Res. 2011, 6, 4726–4735. [Google Scholar] [CrossRef]
- Djidonou, D.; Zhao, X.; Simonne, E.H.; Koch, K.E.; Erickson, J.E. Yield, Water-, and Nitrogen-Use Efficiency in Field-Grown, Grafted Tomatoes. HortScience 2013, 48, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.M.; Romero, L. Nitrogen Efficiency and Metabolism in Grafted Melon Plants. Sci. Hortic. 1999, 81, 113–123. [Google Scholar] [CrossRef]
- Leonardi, C.; Giuffrida, F. Variation of Plant Growth and Macronutrient Uptake in Grafted Tomatoes and Eggplants on Three Different Rootstocks. Eur. J. Hortic. Sci. 2006, 71, 97–101. [Google Scholar]
- Goto, R.; de Miguel, A.; Marsal, J.I.; Gorbe, E.; Calatayud, A. Effect of Different Rootstocks on Growth, Chlorophyll a Fluorescence and Mineral Composition of Two Grafted Scions of Tomato. J. Plant Nutr. 2013, 36, 825–835. [Google Scholar] [CrossRef]
- Frary, A.; Keleş, D.; Pinar, H.; Göl, D.; Doğanlar, S. NaCl Tolerance in Lycopersicon Pennellii Introgression Lines: QTL Related to Physiological Responses. Biol. Plant. 2011, 55, 461–468. [Google Scholar] [CrossRef]
- Ofner, I.; Lashbrooke, J.; Pleban, T.; Aharoni, A.; Zamir, D. Solanum Pennellii Backcross Inbred Lines (BILs) Link Small Genomic Bins with Tomato Traits. Plant J. 2016, 87, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Groher, T.; Schmittgen, S.; Fiebig, A.; Noga, G.; Hunsche, M. Suitability of Fluorescence Indices for the Estimation of Fruit Maturity Compounds in Tomato Fruits. J. Sci. Food Agric. 2018, 98, 5656–5665. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Jpn. Soc. Food Sci. Technol. 1992, 39, 925–928. [Google Scholar] [CrossRef] [Green Version]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. ggplot2. In Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98141-3. [Google Scholar]
- Mancosu, N.; Snyder, R.L.; Kyriakakis, G.; Spano, D. Water Scarcity and Future Challenges for Food Production. Water 2015, 7, 975–992. [Google Scholar] [CrossRef]
- Wang, Q.; Men, L.; Gao, L.; Tian, Y. Effect of Grafting and Gypsum Application on Cucumber (Cucumis Sativus L.) Growth under Saline Water Irrigation. Agric. Water Manag. 2017, 188, 79–90. [Google Scholar] [CrossRef]
- Lee, J.-M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current Status of Vegetable Grafting: Diffusion, Grafting Techniques, Automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Davis, A.R.; Perkins-Veazie, P.; Hassell, R.; Levi, A.; King, S.R.; Zhang, X. Grafting Effects on Vegetable Quality. Am. Soc. Hortic. Sci. 2021, 43, 1670–1672. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Kumar, P.; Chaudhari, S.; Edelstein, M. Tomato Grafting: A Global Perspective. HortScience 2017, 52, 1328–1336. [Google Scholar] [CrossRef] [Green Version]
- Turhan, A.; Ozmen, N.; Serbeci, M.S.; Seniz, V. Effects of Grafting on Different Rootstocks on Tomato Fruit Yield and Quality. Hortic. Sci. 2011, 38, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Rijk-Zwaan. Rijk Zwaan North. America|Product Catalogue Fruitcrops; Rijk Zwaan Canada Leamington Demonstration House: Leamington, ON, Canada, 2020. [Google Scholar]
- Cui, J.; Shao, G.; Lu, J.; Keabetswe, L.; Hoogenboom, G. Yield, Quality and Drought Sensitivity of Tomato to Water Deficit during Different Growth Stages. Sci. Agric. 2020, 77, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Abdulaziz, A.-H.; Abdulrasoul, A.-O.; Thabit, A.; Hesham, A.-R.; Khadejah, A.; Saad, M.; Abdullah, O. Tomato Grafting Impacts on Yield and Fruit Quality under Water Stress Conditions. J. Exp. Biol. Agric. Sci. 2017, 5, 136–147. [Google Scholar] [CrossRef]
- Khah, E.M.; Kakava, E.; Mavromatis, A.; Chachalis, D.; Goulas, C. Effect of Grafting on Growth and Yield of Tomato (Lycopersicon Esculentum Mill.) in Greenhouse and Open-Tield. J. Appl. Hortic. 2006, 8, 3–7. [Google Scholar] [CrossRef]
- Schwarz, D.; Öztekin, G.B.; Tüzel, Y.; Brückner, B.; Krumbein, A. Rootstocks Can Enhance Tomato Growth and Quality Characteristics at Low Potassium Supply. Sci. Hortic. 2013, 149, 70–79. [Google Scholar] [CrossRef]
- Lu, X.; Liu, W.; Wang, T.; Zhang, J.; Li, X.; Zhang, W. Systemic Long-Distance Signaling and Communication between Rootstock and Scion in Grafted Vegetables. Front. Plant Sci. 2020, 11, 460. [Google Scholar] [CrossRef]
- Lacombe, B.; Achard, P. Long-Distance Transport of Phytohormones through the Plant Vascular System. Curr. Opin. Plant Biol. 2016, 34, 1–8. [Google Scholar] [CrossRef]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Vegetable Grafting as a Tool to Improve Drought Resistance and Water Use Efficiency. Front. Plant Sci. 2017, 8, 1130. [Google Scholar] [CrossRef] [Green Version]
- Hemming, S.; de Zwart, F.; Elings, A.; Petropoulou, A.; Righini, I. Cherry Tomato Production in Intelligent Greenhouses—Sensors and AI for Control of Climate, Irrigation, Crop Yield, and Quality. Sensors 2020, 20, 6430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Alseekh, S.; Brotman, Y.; Zheng, Y.; Fei, Z.; Tieman, D.M.; Giovannoni, J.J.; Fernie, A.R.; Klee, H.J. Identification of a Solanum Pennellii Chromosome 4 Fruit Flavor and Nutritional Quality-Associated Metabolite QTL. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talekar, N.S.; Opeña, R.T.; Hanson, P. Helicoverpa Armigera Management: A Review of AVRDC’s Research on Host Plant Resistance in Tomato. Crop. Prot. 2006, 25, 461–467. [Google Scholar] [CrossRef]
- Pogonyi, Á.; Pék, Z.; Helyes, L.; Lugasi, A. Effect of Grafting on the Tomato’s Yield, Quality and Main Fruit Components in Spring Forcing. Acta Aliment. 2005, 34, 453–462. [Google Scholar] [CrossRef]
- Savvas, D.; Savva, A.; Ntatsi, G.; Ropokis, A.; Karapanos, I.; Krumbein, A.; Olympios, C. Effects of Three Commercial Rootstocks on Mineral Nutrition, Fruit Yield, and Quality of Salinized Tomato. J. Plant Nutr. Soil Sci. 2011, 174, 154–162. [Google Scholar] [CrossRef]
- Fallik, E.; Ilic, Z. Grafted Vegetables—The Influence of Rootstock and Scion on Postharvest Quality. Folia Hortic. 2014, 26, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Prudent, M.; Causse, M.; Génard, M.; Tripodi, P.; Grandillo, S.; Bertin, N. Genetic and Physiological Analysis of Tomato Fruit Weight and Composition: Influence of Carbon Availability on QTL Detection. J. Exp. Bot. 2009, 60, 923–937. [Google Scholar] [CrossRef] [Green Version]
- Misković, A.; Ilin, Z.; Marković, V. Effect of Different Rootstock Type on Quality and Yield of Tomato Fruits. Acta Hortic. 2009, 807, 619–624. [Google Scholar] [CrossRef]
- Riga, P.; Benedicto, L.; García-Flores, L.; Villaño, D.; Medina, S.; Gil-Izquierdo, Á. Rootstock Effect on Serotonin and Nutritional Quality of Tomatoes Produced under Low Temperature and Light Conditions. J. Food Compos. Anal. 2016, 46, 50–59. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Qu, Z.; Wang, S.; He, P.; Zhang, N. Effects of Alternating Irrigation with Fresh and Saline Water on the Soil Salt, Soil Nutrients, and Yield of Tomatoes. Water 2019, 11, 1693. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Du, T.; Mao, X.; Ding, R.; Shukla, M.K. A Comprehensive Method of Evaluating the Impact of Drought and Salt Stress on Tomato Growth and Fruit Quality Based on EPIC Growth Model. Agric. Water Manag. 2019, 213, 116–127. [Google Scholar] [CrossRef]
- Kraska, T.; Kleinschmidt, B.; Weinand, J.; Pude, R. Cascading Use of Miscanthus as Growing Substrate in Soilless Cultivation of Vegetables (Tomatoes, Cucumbers) and Subsequent Direct Combustion. Sci. Hortic. 2018, 235, 205–213. [Google Scholar] [CrossRef]
- FAO. Good Agricultural Practices for Greenhouse Vegetable Crops: Principles for Mediterranean Climate Areas; FAO Plant Production and Protection Paper; Food and Agricultural Organization of the United Nations (FAO): Rome, Italy, 2013; ISBN 978-92-5-107650-7. [Google Scholar]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving Environmentally Sustainable Growing Media for Soilless Plant Cultivation Systems—A Review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellenberger, J.; Bulut, A.; Blömeke, P.; Röhlen-Schmittgen, S. Novel S. pennellii × S. lycopersicum Hybrid Rootstocks for Tomato Production with Reduced Water and Nutrient Supply. Horticulturae 2021, 7, 355. https://doi.org/10.3390/horticulturae7100355
Ellenberger J, Bulut A, Blömeke P, Röhlen-Schmittgen S. Novel S. pennellii × S. lycopersicum Hybrid Rootstocks for Tomato Production with Reduced Water and Nutrient Supply. Horticulturae. 2021; 7(10):355. https://doi.org/10.3390/horticulturae7100355
Chicago/Turabian StyleEllenberger, Jan, Aylin Bulut, Philip Blömeke, and Simone Röhlen-Schmittgen. 2021. "Novel S. pennellii × S. lycopersicum Hybrid Rootstocks for Tomato Production with Reduced Water and Nutrient Supply" Horticulturae 7, no. 10: 355. https://doi.org/10.3390/horticulturae7100355
APA StyleEllenberger, J., Bulut, A., Blömeke, P., & Röhlen-Schmittgen, S. (2021). Novel S. pennellii × S. lycopersicum Hybrid Rootstocks for Tomato Production with Reduced Water and Nutrient Supply. Horticulturae, 7(10), 355. https://doi.org/10.3390/horticulturae7100355