State of the Art and Elucidation of Postharvest LED Lighting on the Metabolism of Brassica Sprouts
Abstract
:1. Introduction
2. Materials and Methods
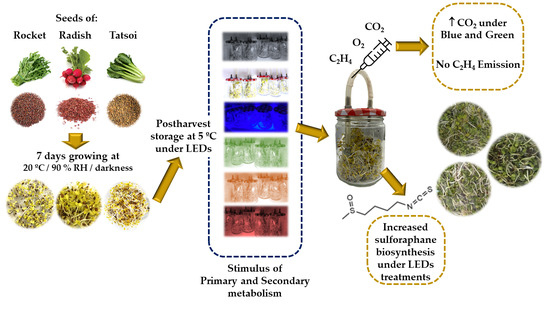
2.1. Plant Material, Seed Germination, and Minimal Processing
2.2. Postharvest Light Treatments
- ▪
- Darkness: used as control
- ▪
- White (400–650 nm; 5000–6500 K CCT and ~96% CRI) LED: 20.6 W m−2 = 1779.8 kJm−2 d−1 = 74.2 kJm−2 h−1
- ▪
- Blue (435 nm) LED: 20.5 W m−2 = 1771.2 kJm−2 d−1 = 73.8 kJm−2 h−1
- ▪
- Green (500 nm) LED: 20.4 W m−2 = 1762.6 kJm−2 d−1 = 73.4 kJm−2 h−1
- ▪
- Orange (610 nm) LED: 20.5 W m−2 = 1771.2 kJm−2 d−1 = 73.8 kJm−2 h−1
- ▪
- Red (660 nm) LED: 21 W m−2 = 1814.4 kJm−2 d−1 = 75.6 kJm−2 h−1
2.3. Respiration Rate and Ethylene Production
2.4. Sulforaphane Extraction and Analysis
2.5. Scientific Literature Review
2.6. Statistical Analysis
3. Results
3.1. Postharvest Sprouts Characterisation
3.2. Influence of LED Lighting on the Primary Metabolism of Brassicaceae Sprouts
3.3. Influence of LED Lighting on the Secondary Metabolism of Brassicaceae Sprouts
4. Discussion
| Specie | Light Treatment | Primary Metabolism Parameter Tested | Main Findings | Ref. |
|---|---|---|---|---|
| Brussels sprouts | Sunlight | Respiration and photosynthesis Color retention | Increase of the respiration and the photosynthesis rates. High color retention under light | [38] |
| Soybean sprouts | 5 min pulse of white light 350 µmol m−2 s−1 every 12 h | Respiration Total soluble sugars Starch and chlorophylls | Slight increase of O2 release at the beginning. Soluble sugars, starch concentrations, and chlorophyll increased after illumination | [42] |
| Pakchoi | White LED 10 μmol m−2 s−1 | Respiration rate Chlorophyll and vitamin C | Respiration rate was lower under LED lighting. LED delayed senescence at 20 °C | [39] |
| Mung bean sprouts | Pulsed light (17–25% UV) from 200 to 1100 nm at 0.1 and 1.0 J cm−2 | Color, respiration rates | No increase in respiration rates, but a positive impact on quality | [45] |
| Radish, soybean, mung bean, and pumpkin sprouts | White, Blue, and Red LED 110 μmol m−2 s−1 | Dry matter Soluble carbohydrates and starch | The soluble sugars and starch increased under LED lighting in radish and pumpkin, but not in mung bean and soybean. | [46] |
| Kale sprouts | White, Blue, Red, and Far-Red LED 1–100 μmol m−2 s−1 | Growth | The growth was higher under Darkness conditions, followed by White, Red, Blue, and Far-Red LED lighting. | [47] |
| Onion bulbs | White LED 100 μmol m−2 s−1 and UV lamp at 254 nm | Sugar concentration | UV light reduced fructose and glucose concentrations | [48] |
| Turnip, Cauliflower, and Mustard sprouts | Seeds treated with laser (632 nm, Red) at 5 mW for 5 min and 500 mJ energy | Photosynthesis and respiration Fresh weight | Photosynthesis, respiration, and total weight was increased | [49] |
| Lemongrass sprouts | Seeds treated with lasers (632 nm, Red) at 5 mW for 5 min and 500 mJ energy | Photosynthesis Sugar, amino acid, organic acid, and essential oil analysis | Increase in photosynthesis, respiration, and fresh weight. The synthesis of primary metabolites as amino acids, organic acids, and essential oils was also increased | [50] |
| Pakchoi | White LED 30 μmol m−2 s−1 | Respiration rate Soluble sugars | The combination of LED and MAP reduced the respiration rate during 15 days of storage at 20 °C. It also increased the soluble sugars content. | [40] |
| Broccoli sprouts | White, Red, Yellow, Green, Blue, and Purple LEDs 60 μmol m−2 s−1 | Growth Sugar content | LEDs inhibited the sprout growth. Red and Green LEDs decreased sugar content, while White, Blue, and Purple increased fructose content | [51] |
| Specie | Light Treatment | Secondary Metabolism Parameter Tested | Main Findings | Ref. |
|---|---|---|---|---|
| Pakchoi | White LED 10 μmol m−2 s−1 | Chlorophyll, vitamin C, and antioxidant enzyme activity | Chlorophyll, vitamin C, and enzymatic activity were increased | [39] |
| Kale sprouts | White, Blue, Red, and Far-Red LED 1–100 μmol m−2 s−1 | Chlorophylls, anthocyanins, glucosinolates, and total antioxidant capacity | LED lighting increased the antioxidant capacity and secondary metabolites assessed | [47] |
| Broccoli sprouts | Red and Blue LEDs 350 and 41 μmol m−2 s−1 | Chlorophylls, carotenoids, and glucosinolates | Chlorophylls, carotenoids, and glucoraphanin were highly biosynthesized after Blue LED treatments | [55] |
| Broccoli sprouts | Red, Green, and Blue LEDs 250 μmol m−2 s−1 | Chlorophylls, carotenoids, and glucosinolates | Secondary metabolites were highly biosynthesized under Blue LEDs | [56] |
| Onion bulbs | UV laser treatment and White LED 100 μmol m−2 s−1 | Quercetin glycosides | Quercetin glycosides concentrations increased the most when exposed to UV light and, to a lesser extent, when exposed to visible light | [48] |
| Radish, soybean, mung bean, and pumpkin sprouts | White, Blue, and Red LED 110 μmol m−2 s−1 | Polyphenols, chlorophyll, carotenoids, vitamin C, and anthocyanins | Vitamin C, anthocyanins, carotenoids, and chlorophylls increased under White, Blue, and Red LEDs, but total phenolic content was maintained | [46] |
| Turnip, Cauliflower, and Mustard sprouts | Seeds were treated with He−Ne laser (632 nm; 5 mW; 5 min; 500 mJ) | Chlorophylls, carotenoids, phenolic compounds, glucosinolates, and sulforaphane | Laser treatment on seeds before sowing increased the chlorophylls, carotenoids, total glucosinolates, glucoraphanin, and sulforaphane contents, and myrosinase activity | [49] |
| Lemongrass sprouts | Seeds were treated with lasers (632 nm; Red; 5 mW; 5 min; 500 mJ) | Phenolic compounds and antioxidant capacity | Laser treatment on seeds improved the synthesis of phenolic compounds and antioxidant capacity | [50] |
| Pakchoi | White LED 30 μmol m−2 s−1 | Ascorbic acid, chlorophylls and antioxidant capacity | LED + MAP reduced the degradation of ascorbic acid and chlorophylls, increasing the antioxidant capacity | [40] |
| Broccoli sprouts | Blue, Red, and Far-Red LEDs 35 μmol m−2 s−1 | Phenolic compounds | LED lighting increased the biosynthesis of phenolics | [31] |
| Broccoli sprouts | White, Yellow, and Green LEDs 35 μmol m−2 s−1 | Phenolic compounds and glucosinolates | Yellow LED lighting increased the biosynthesis of phenolics and glucosinolates | [24] |
| Rocket sprouts | Photoperiod of 14 h 32 μmol m−2 s−1 Fluorescent light + 10 h 47.3 μmol m−2 s−1 White, Red, or Blue LEDs | Phenolic acids, flavonoids, glucosinolates, and sulforaphane | The application of White, Blue, Red LEDs for 10 h enhanced the biosynthesis of sulforaphane, glucosinolates, and phenolic compounds. | [56] |
| Broccoli leaves | Red and Blue LEDs 200 μmol m−2 s−1 | Glucosinolates and sulforaphane | Red LEDs promoted glucosinolates biosynthesis and sulforaphane accumulation, whereas Blue LEDs inhibited this effect | [53] |
| Broccoli sprouts | White, Red, Yellow, Green, Blue, and Purple LEDs 60 μmol m−2 s−1 | Anthocyanins and ascorbic acid content Glucosinolates and sulforaphane | Yellow, Blue, and Purple LEDs increased glucoraphanin and anthocyanins contents. All the LED treatments increased ascorbic acid and sulforaphane contents. | [51] |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, T.; Cole, M.; Farber, J.M.; Eisenbrand, G.; Zabaras, D.; Fox, E.M.; Hill, J.P. Food Safety for Food Security: Relationship between Global Megatrends and Developments in Food Safety. Trends Food Sci. Technol. 2017, 68, 160–175. [Google Scholar] [CrossRef]
- Barba, F.J.; Mariutti, L.R.B.; Bragagnolo, N.; Mercadante, A.Z.; Barbosa-Cánovas, G.V.; Orlien, V. Bioaccessibility of Bioactive Compounds from Fruits and Vegetables after Thermal and Nonthermal Processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and Vegetable Waste Management and the Challenge of Fresh-Cut Salad. Trends Food Sci. Technol. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and Microgreens: Trends, Opportunities, and Horizons for Novel Research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Johnson, T.L.; Dinkova-Kostova, A.T.; Fahey, J.W. Glucosinolates from the Brassica Vegetables and Their Health Effects; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9780123849533. [Google Scholar]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Genotypic Effects on the Phytochemical Quality of Seeds and Sprouts from Commercial Broccoli Cultivars. Food Chem. 2011, 125, 348–354. [Google Scholar] [CrossRef]
- Abellán, A.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Sorting out the Value of Cruciferous Sprouts as Sources of Bioactive Compounds for Nutrition and Health. Nutrients 2019, 11, 429. [Google Scholar] [CrossRef] [Green Version]
- Bell, L.; Wagstaff, C. Rocket Science: A Review of Phytochemical & Health-Related Research in Eruca & Diplotaxis Species. Food Chem. X 2019, 1, 100002. [Google Scholar] [CrossRef] [PubMed]
- Gamba, M.; Asllanaj, E.; Raguindin, P.F.; Glisic, M.; Franco, O.H.; Minder, B.; Bussler, W.; Metzger, B.; Kern, H.; Muka, T. Nutritional and Phytochemical Characterization of Radish (Raphanus sativus): A Systematic Review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional Quality and Health Benefits of Microgreens, a Crop of Modern Agriculture. J. Futur. Foods 2021, 1, 58–66. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional Quality in Novel Food Sources: Genotypic Variation in the Nutritive and Phytochemical Composition of Thirteen Microgreens Species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived from the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef] [Green Version]
- McCree, K.J. The Action Spectrum, Absorptance and Quantum Yield of Photosynthesis in Crop Plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- McCree, K.J. Test of Current Definitions of Photosynthetically Active Radiation against Leaf Photosynthesis Data. Agric. Meteorol. 1972, 10, 443–453. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development and Metabolism; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Angelova, Z.; Georgiev, S.; Roos, W. Elicitation of Plants. Biotechnol. Biotechnol. Equip. 2006, 20, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Poulev, A.; O’Neal, J.M.; Logendra, S.; Pouleva, R.B.; Timeva, V.; Garvey, A.S.; Gleba, D.; Jenkins, I.S.; Halpern, B.T.; Kneer, R.; et al. Elicitation, a New Window into Plant Chemodiversity and Phytochemical Drug Discovery. J. Med. Chem. 2003, 46, 2542–2547. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A Review on the Effects of Light-Emitting Diode (LED) Light on the Nutrients of Sprouts and Microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Vitale, E.; Velikova, V.; Tsonev, T.; Ferrandino, I.; Capriello, T.; Arena, C. The Interplay between Light Quality and Biostimulant Application Affects the Antioxidant Capacity and Photosynthetic Traits of Soybean (Glycine max L. Merrill). Plants 2021, 10, 861. [Google Scholar] [CrossRef]
- Pennisi, G.; Sanyé-Mengual, E.; Orsini, F.; Crepaldi, A.; Nicola, S.; Ochoa, J.; Fernandez, J.A.; Gianquinto, G. Modelling Environmental Burdens of Indoor-Grown Vegetables and Herbs as Affected by Red and Blue LED Lighting. Sustainability 2019, 11, 4063. [Google Scholar] [CrossRef] [Green Version]
- Pennisi, G.; Orsini, F.; Castillejo, N.; Gómez, P.A.; Crepaldi, A.; Fernández, J.A.; Egea-Gilabert, C.; Artés-Hernández, F.; Gianquinto, G. Spectral Composition from Led Lighting during Storage Affects Nutraceuticals and Safety Attributes of Fresh-Cut Red Chard (Beta vulgaris) and Rocket (Diplotaxis tenuifolia) Leaves. Postharvest Biol. Technol. 2021, 175, 111500. [Google Scholar] [CrossRef]
- Janssen, R.J.P.; Krijn, M.P.C.M.; van den Bergh, T.; van Elmpt, R.F.M.; Nicole, C.C.S.; van Slooten, U. Optimizing Plant Factory Performance for Local Requirements. In Plant Factory Using Artificial Light: Adapting to Environmental Disruption and Clues to Agricultural Innovation; Anpo, M., Fukuda, H., Wada, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 281–293. ISBN 9780128139745. [Google Scholar]
- Samuolienė, G.; Brazaitytė, A.; Viršilė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Duchovskis, P. Nutrient Levels in Brassicaceae Microgreens Increase Under Tailored Light-Emitting Diode Spectra. Front. Plant Sci. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Castillejo, N.; Martínez-Zamora, L.; Gómez, P.A.; Pennisi, G.; Orsini, F.; Art, F.; Crepaldi, A.; Fernández, J.A.; Artés-Hernández, F. Postharvest Yellow LED Lighting Affects Phenolics and Glucosinolates Biosynthesis in Broccoli Sprouts. J. Food Compos. Anal. 2021, 103, 104101. [Google Scholar] [CrossRef]
- Hernández-Cánovas, L.; Abellán-Victorio, Á.; Moreno, D.A. The Quality and Glucosinolate Composition of Cruciferous Sprouts under Elicitor Treatments Using MeJA and LED Lights. Proceedings 2020, 70, 67. [Google Scholar] [CrossRef]
- Han, T.; Astafurova, T.; Turanov, S.; Burenina, A.; Butenkova, A.; Surnina, E.; Valiev, D. Photomorphogenesis of Wheat Sprouts with LED Irradiation of Different Intensities. Light. Res. Technol. 2020, 52, 583–594. [Google Scholar] [CrossRef]
- Giménez, A.; Martínez, C.; Egea-Gilabert, C.; Gómez, P.A.; Artés-Hernández, F.; Pennisi, G.; Orsini, F.; Crepaldi, A.; Fernández, J.A. Combined Effect of Salinity and LED Lights on the Yield and Quality of Purslane (Portulaca oleracea L.) Microgreens. Horticulturae 2021, 7, 180. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Castillejo, N.; Gómez, P.A.; Artés-Hernández, F. Amelioration Effect of Led Lighting in the Bioactive Compounds Synthesis during Carrot Sprouting. Agronomy 2021, 11, 304. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, N.S.; Park, J.S.; Lee, S.Y.; Lee, J.W.; Park, S.U. Effects of Light-Emitting Diodes on the Accumulation of Glucosinolates and Phenolic Compounds in Sprouting Canola (Brassica napus L.). Foods 2019, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Postharvest UV-B and UV-C Radiation Enhanced the Biosynthesis of Glucosinolates and Isothiocyanates in Brassicaceae Sprouts. Postharvest Biol. Technol. 2021, 181, 111650. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Gómez, P.A.; Pennisi, G.; Crepaldi, A.; Fernández, J.A.; Orsini, F.; Artés-Hernández, F. Postharvest LED Lighting: Effect of Red, Blue and Far Red on Quality of Minimally Processed Broccoli Sprouts. J. Sci. Food Agric. 2021, 101, 44–53. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Artés–Hernández, F. Periodical Uv-B Radiation Hormesis in Biosynthesis of Kale Sprouts Nutraceuticals. Plant Physiol. Biochem. 2021, 165, 274–285. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Castillejo, N.; Artés–Hernández, F. UV-B Radiation as Abiotic Elicitor to Enhance Phytochemicals and Development of Red Cabbage Sprouts. Horticulturae 2021, 7, 567. [Google Scholar] [CrossRef]
- Artés–Hernández, F.; Miranda-Molina, F.D.; Klug, T.V.; Martínez–Hernández, G.B. Enrichment of Glucosinolate and Carotenoid Contents of Mustard Sprouts by Using Green Elicitors during Germination. J. Food Compos. Anal. 2022, 110, 104546. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Avalos-Belmontes, F.; Rodríguez-Hernández, A.M.; Castillo-Campohermoso, M.A.; Artés-Hernández, F. An Innovative Ethylene Scrubber Made of Potassium Permanganate Loaded on a Protonated Montmorillonite: A Case Study on Blueberries. Food Bioprocess Technol. 2019, 12, 524–538. [Google Scholar] [CrossRef]
- Livadariu, O.; Maximilian, C.; Raiciu, A.D.; Șerbănică, C.P. An Assessment of the Type of Substrate and LEDs’ Irradiation Influence on Garden Cress Sprouts (Lepidium sativum L.). Appl. Sci. 2022, 12, 4732. [Google Scholar] [CrossRef]
- Korzeniewska, E.; Czarnecki, A.; Kiełbasa, P. Modelling of Carotenoids Content in Red Clover Sprouts Using Light of Di Ff Erent Wavelength and Pulsed Electric Field. Appl. Sci. 2020, 10, 4143. [Google Scholar] [CrossRef]
- Eaves, C.A.; Forsyth, F.R. The Influence of Light, Modified Atmospheres and Benzimidazole on Brussels Sprouts. J. Hortic. Sci. 1968, 43, 317–322. [Google Scholar] [CrossRef]
- Zhou, F.; Zuo, J.; Xu, D.; Gao, L.; Wang, Q.; Jiang, A. Low Intensity White Light-Emitting Diodes (LED) Application to Delay Senescence and Maintain Quality of Postharvest Pakchoi (Brassica campestris L. Ssp. Chinensis (L.) Makino Var. Communis Tsen et Lee). Sci. Hortic. 2020, 262, 109060. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhang, M.; Chitrakar, B.; Devahastin, S.; Guo, Z. Novel Combined Use of Red-White LED Illumination and Modified Atmosphere Packaging for Maintaining Storage Quality of Postharvest Pakchoi. Food Bioprocess Technol. 2022, 15, 590–605. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Wang, J.; Yao, Y.; Hu, H. Respiration Rate Measurement and Chemical Kinetic Modelling for Mung Bean Sprouts. J. Food Process Eng. 2017, 40, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ribas-Carbo, M.; Robinson, S.A.; Gonzàlez-Meler, M.A.; Lennon, A.M.; Giles, L.; Siedow, J.N.; Berry, J.A. Effects of Light on Respiration and Oxygen Isotope Fractionation in Soybean Cotyledons. Plant Cell Environ. 2000, 23, 983–989. [Google Scholar] [CrossRef] [Green Version]
- Young-Sang, L.; Young-Ho, K. Changes in Post-Harvest Respiration, Growth and Vitamin C Content of Soybean Sprouts under Different Storage Temperature Conditions. Korean J. Crop Sci. 2004, 49, 410–414. [Google Scholar]
- Singh, R.; Kumar, A.; Mann, S.; Kulkarni, S.D. Respiration Rate Models of Fresh Chickpea Sprouts (Cicer arietinum L.) under Modified Atmosphere Packaging. Agric. Eng. 2012, 37, 49–57. [Google Scholar]
- Kramer, B.; Wunderlich, J.; Muranyi, P. Pulsed Light Decontamination of Endive Salad and Mung Bean Sprouts and Impact on Color and Respiration Activity. J. Food Prot. 2015, 78, 340–348. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Dipierro, N.; Paciolla, C. Effects of Darkness and Light Spectra on Nutrients and Pigments in Radish, Soybean, Mung Bean and Pumpkin Sprouts. Antioxidants 2020, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.D.; Folta, K.M. Sequential Light Programs Shape Kale (Brassica napus) Sprout Appearance and Alter Metabolic and Nutrient Content. Hortic. Res. 2014, 1, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, K.S.; Lee, E.J.; Patil, B.S. Changes in Quercetin Glucoside Concentrations of Onion Bulbs by Scales, during Storage, and in Sprouting Leaves Exposed to UV. Postharvest Biol. Technol. 2013, 83, 65–71. [Google Scholar] [CrossRef]
- Almuhayawi, S.M.; Almuhayawi, M.S.; Al Jaouni, S.K.; Selim, S.; Hassan, A.H.A. Effect of Laser Light on Growth, Physiology, Accumulation of Phytochemicals, and Biological Activities of Sprouts of Three Brassica Cultivars. J. Agric. Food Chem. 2021, 69, 6240–6250. [Google Scholar] [CrossRef] [PubMed]
- Okla, M.K.; El-Tayeb, M.A.; Qahtan, A.A.; Abdel-Maksoud, M.A.; Elbadawi, Y.B.; Alaskary, M.K.; Balkhyour, M.A.; Hassan, A.H.A.; Abdelgawad, H. Laser Light Treatment of Seeds for Improving the Biomass Photosynthesis, Chemical Composition and Biological Activities of Lemongrass Sprouts. Agronomy 2021, 11, 478. [Google Scholar] [CrossRef]
- Zhuang, L.; Huang, G.; Li, X.; Xiao, J.; Guo, L. Effect of Different LED Lights on Aliphatic Glucosinolates Metabolism and Biochemical Characteristics in Broccoli Sprouts. Food Res. Int. 2022, 154, 111015. [Google Scholar] [CrossRef]
- Obata, T. Metabolons in Plant Primary and Secondary Metabolism. Phytochem. Rev. 2019, 18, 1483–1507. [Google Scholar] [CrossRef]
- Wang, J.; Mao, S.; Wu, Q.; Yuan, Y.; Liang, M.; Wang, S.; Huang, K.; Wu, Q. Effects of LED Illumination Spectra on Glucosinolate and Sulforaphane Accumulation in Broccoli Seedlings. Food Chem. 2021, 356, 129550. [Google Scholar] [CrossRef]
- Jin, P.; Yao, D.; Xu, F.; Wang, H.; Zheng, Y. Effect of Light on Quality and Bioactive Compounds in Postharvest Broccoli Florets. Food Chem. 2015, 172, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Kopsell, D.A.; Sams, C.E. Increases in Shoot Tissue Pigments, Glucosinolates, and Mineral Elements in Sprouting Broccoli after Exposure to Short-Duration Blue Light from Light Emitting Diodes. J. Am. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting Broccoli Accumulate Higher Concentrations of Nutritionally Important Metabolites under Narrow-Band Light-Emitting Diode Lighting. J. Am. Soc. Hortic. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Zamora, L.; Castillejo, N.; Artés–Hernández, F. Ultrasounds and a Postharvest Photoperiod to Enhance the Synthesis of Sulforaphane and Antioxidants in Rocket Sprouts. Antioxidants 2022, 11, 1490. [Google Scholar] [CrossRef] [PubMed]





| Rocket | Radish | Tatsoi | |
|---|---|---|---|
| Fresh weight/Dry weight (FW/DW) | 11.75 ± 0.83 a | 7.20 ± 0.79 b | 9.82 ± 1.24 a |
| % Moisture | 91.46 ± 0.59 a | 85.99 ± 1.63 b | 89.72 ± 1.25 a |
| Hypocotyl length (mm) | 32.7 ± 3.0 b | 50.5 ± 5.5 a | 25.6 ± 3.8 b |
| Root length (mm) | 28.7 ± 0.1 b | 36.5 ± 4.3 a | 25.9 ± 4.8 b |
| Sprout length (mm) | 61.4 ± 3.0 b | 86.9 ± 9.2 a | 51.5 ± 1.5 c |
| Growth rate (mm/day) | 4.68 ± 0.43 b | 7.21 ± 0.79 a | 3.65 ± 0.54 c |
| Visual appearance |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Zamora, L.; Castillejo, N.; Cano-Lamadrid, M.; Artés-Hernández, F. State of the Art and Elucidation of Postharvest LED Lighting on the Metabolism of Brassica Sprouts. Horticulturae 2022, 8, 1065. https://doi.org/10.3390/horticulturae8111065
Martínez-Zamora L, Castillejo N, Cano-Lamadrid M, Artés-Hernández F. State of the Art and Elucidation of Postharvest LED Lighting on the Metabolism of Brassica Sprouts. Horticulturae. 2022; 8(11):1065. https://doi.org/10.3390/horticulturae8111065
Chicago/Turabian StyleMartínez-Zamora, Lorena, Noelia Castillejo, Marina Cano-Lamadrid, and Francisco Artés-Hernández. 2022. "State of the Art and Elucidation of Postharvest LED Lighting on the Metabolism of Brassica Sprouts" Horticulturae 8, no. 11: 1065. https://doi.org/10.3390/horticulturae8111065
APA StyleMartínez-Zamora, L., Castillejo, N., Cano-Lamadrid, M., & Artés-Hernández, F. (2022). State of the Art and Elucidation of Postharvest LED Lighting on the Metabolism of Brassica Sprouts. Horticulturae, 8(11), 1065. https://doi.org/10.3390/horticulturae8111065