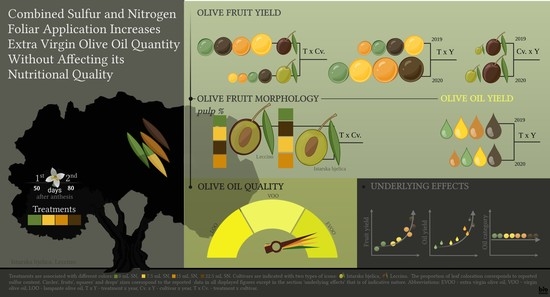
Combined Sulfur and Nitrogen Foliar Application Increases Extra Virgin Olive Oil Quantity without Affecting Its Nutritional Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions, Treatments, and Sampling
2.2. Soil Characterization
2.3. Leaf Sampling
2.4. Fruit Sampling
2.5. Chemicals
2.6. S and N Concentration Measurements
2.7. Oil Extraction
2.8. Determination of Water and Oil Content
2.9. Olive Oil Yields
2.10. Free Fatty Acids (FFA), Peroxide Value (PV), and Extinction Coefficients Measurements
2.11. TPC in Olive Oil
2.12. FAME Determination
2.13. Statistical Analysis
2.14. Graphical Content
3. Results
3.1. S and N Concentration in Olive Leaves
3.2. Fruit Yield
3.3. Fruit Morphology
3.4. Moisture, Dry Matter, and Oil Content in Olive Paste, Oil Extraction Yield, and Oil Yield per Tree
3.5. Quality Parameters
3.6. Fatty Acids
4. Discussion
4.1. S and N Concentration in Olive Leaves
4.2. Fruit Yield
4.3. Fruit Morphology
4.4. Moisture, Dry Matter, and Oil Content
4.5. Oil Yields
4.6. Quality Parameters
4.7. TPC
4.8. Fatty Acids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brito, C.; Dinis, L.T.; Moutinho-Pereira, J.; Correia, C.M. Drought stress effects and olive tree acclimation under a changing climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef] [Green Version]
- Alves, E.; Melo, T.; Barros, M.P.; Domingues, M.M.R.; Domingues, P. Lipidomic Profiling of the Olive (Olea europaea L.) Fruit towards Its Valorisation as a Functional Food: In-depth identification of triacylglycerols and polar lipids in Portuguese olives. Molecules 2019, 24, 2555. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Abdin, M.Z. Effect of sulphur application on lipid, RNA and fatty acid content in developing seeds of rapeseed (Brassica campestris L.). Plant Sci. 2000, 150, 71–76. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Factors influencing phenolic compounds in table olives (Olea europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef]
- Brighente, I.M.C.; Dias, M.; Verdi, L.G.; Pizzolatti, M.G. Antioxidant activity and total phenolic content of some Brazilian species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Malhi, S.S. Interactions of Nitrogen with Other Nutrients and Water: Effect on Crop Yield and Quality, Nutrient Use Efficiency, Carbon Sequestration, and Environmental Pollution. Adv. Agron. 2005, 86, 341–409. [Google Scholar] [CrossRef]
- Malhi, S.S.; Gill, K.S. Effectiveness of sulphate-S fertilization at different growth stages for yield, seed quality and S uptake of canola. Can. J. Plant Sci. 2002, 82, 665–674. [Google Scholar] [CrossRef]
- Pasricha, N.S.; Sahota, N.S. Yield, nutrient concentration and quality of mustard crops as influenced by nitrogen and sulphur fertilizers. J. Agric. Sci. 1980, 94, 545–549. [Google Scholar] [CrossRef]
- Chowdhury, M.A.H.; Sultana, T.; Rahman, M.A.; Saha, B.K.; Chowdhury, T.; Tarafder, S. Sulphur fertilization enhanced yield, its uptake, use efficiency and economic returns of Aloe vera L. Heliyon 2020, 6, e05726. [Google Scholar] [CrossRef]
- Brosnan, J.; Brosnan, M.E. Amino Acid Assessment Workshop. J. Nutr. 2006, 136, 16365–16405. [Google Scholar]
- Ergle, D.R.; Eaton, M. Sulphur nutrition of Aloe vera plants. J. Plant Physiol. 2005, 26, 639–6546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Šegota, T.; Filipčić, A. Köppen’s classification of climates and the problem of corresponding Croatian terminology. Geoadria 2003, 8, 17–37. [Google Scholar] [CrossRef] [Green Version]
- HRN ISO 10390; Kakvoća tla–Određivanje pH-Vrijednosti (ISO 10390:2005). Hrvatski Zavod za Norme: Zagreb, Hrvatska, 2005.
- HRN ISO 11261; Kakvoća tla–Određivanje Ukupnog Dušika–Prilagođena Kjedahlova Metoda (ISO 11261:1995). Hrvatski Zavod za Norme: Zagreb, Hrvatska, 2004.
- ISO 14235; Soil Quality—Determination of Organic Carbon by Sulfochromic Oxidation. International Organization for Standardization: Genève, Switzerland, 1998.
- HRN ISO 10693; Kakvoća tla–Određivanje Sadržaja Karbonata-Volumetrijska Metoda (ISO 10693:1995). Hrvatski Zavod za Norme: Zagreb, Hrvatska, 2004.
- Egner, H.; Riehm, H.; Domingo, W.R. Untersuchung über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nahrstoffzustanden der Boden. II. Chemische Extraktionsmethoden zur Phosphor und Kaliumbestimmung. K. LantbR. HogsK. AnniR. W. R. 1960, 26, 199–215. [Google Scholar]
- Pasković, I.; Herak Ćustić, M.; Pecina, M.; Bronić, J.; Ban, D.; Radić, T.; Pošćić, F.; Jukić Špika, M.; Soldo, B.; Palčić, I.; et al. Manganese soil and foliar fertilization of olive plantlets: The effect on leaf mineral and phenolic content and root mycorrhizal colonization. J. Sci. Food Agric. 2019, 99, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Uceda, M.; Frias, L. Evolution of the Fruit Oil Content, Oil Composition and Oil Quality. In Proceedings of the II Seminario Oleícola Internacional; International Olive Oil Council: Cordoba, Spain, 1975; p. 125130. [Google Scholar]
- Guzmán, E.; Baeten, V.; Pierna, J.A.F.; García-Mesa, J.A. Determination of the olive maturity index of intact fruits using image analysis. J. Food Sci. Technol. 2015, 52, 1462–1470. [Google Scholar] [CrossRef] [Green Version]
- Barranco, D.; Cimato, A.; Fiorino, P.; Rallo, L.; Touzani, A.; Castaneda, C.; Serafini, F.; Trujillo, I. World Catalogue of Olive Varietes; International Olive Oil Council: Madrid, Spain, 2000. [Google Scholar]
- Vidović, N.; Pasković, I.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Grozić, K.; Cukrov, M.; Marcelić, Š.; Ban, D.; Talhaoui, N.; et al. Biophenolic profile modulations in olive tissues as affected by manganese nutrition. Plants 2021, 10, 1724. [Google Scholar] [CrossRef]
- HRN ISO 660; Životinjske i Biljne Masti i Ulja–Određivanje Kiselinskog Broja i Kiselosti (ISO 660:2009; EN ISO 660:2009). Hrvatski Zavod za Norme: Zagreb, Hrvatska, 2010.
- HRN ISO 3960; Životinjske i Biljne Masti i Ulja–Određivanje Peroksidnog Broja–Jodometrijsko Određivanje Točke Završetka (ISO 3960:2007, Ispravljena Verzija 2009-05-15; EN ISO 3960:2010). Hrvatski Zavod za Norme: Zagreb, Hrvatska, 2010.
- HRN ISO 3656; Životinjske i Biljne Masti i Ulja–Određivanje Apsorbancija u Ultraljubičastom Spektru Izraženih kao Specifična UV Ekstinkcija (ISO 3656:2011; EN ISO 3656:2011). Hrvatski Zavod za Norme: Zagreb, Hrvatska, 2011.
- International Olive Council. Determination of Fatty Acid Methyl Esters by Gas Chromatography. COI/T.20/Doc. No 33/Rev. 1. 2017. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T.20-Doc.-No-33-Rev.-1-2017.pdf. (accessed on 31 December 2021).
- Fernández-Escobar, R.; Moreno, R.; García-Creus, M. Seasonal changes of mineral nutrients in olive leaves during the alternate-bearing cycle. Sci. Hortic. (Amst.) 1999, 82, 25–45. [Google Scholar] [CrossRef]
- Carciochi, W.D.; Salvagiotti, F.; Pagani, A.; Reussi Calvo, N.I.; Eyherabide, M.; Sainz Rozas, H.R.; Ciampitti, I.A. Nitrogen and sulfur interaction on nutrient use efficiencies and diagnostic tools in maize. Eur. J. Agron. 2020, 116, 126045. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; García-Novelo, J.M.; Restrepo-Diaz, H. Mobilization of nitrogen in the olive bearing shoots after foliar application of urea. Sci. Hortic. 2011, 127, 452–454. [Google Scholar] [CrossRef]
- Perica, S.; Androulakis, I.I.; Loupassaki, M.H. Effect of Summer Application of Nitrogen and Potassium on Mineral Composition of Olive Leaves. Acta Hortic. 1994, 356, 221–224. [Google Scholar] [CrossRef]
- Jan, A.; Ahmad, G.; Arif, M.; Jan, M.T.; Marwat, K.B. Quality parameters of canola as affected by nitrogen and sulfur fertilization. J. Plant Nutr. 2010, 33, 381–390. [Google Scholar] [CrossRef]
- Zipori, I.; Erel, R.; Yermiyahu, U.; Ben-gal, A.; Dag, A. Sustainable management of olive orchard nutrition: A review. Agriculture 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Escobar, R. Olive Nutritional Status and Tolerance to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Chatzissavvidis, C.; Therios, I. Response of four olive (Olea europaea L.) cultivars to six B concentrations: Growth performance, nutrient status and gas exchange parameters. Sci. Hortic. (Amst.) 2010, 127, 29–38. [Google Scholar] [CrossRef]
- Pasković, I.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D.; et al. Temporal variation of phenolic and mineral composition in olive leaves is cultivar dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef]
- Lukić, I.; Pasković, I.; Žurga, P.; Majetić Germek, V.; Brkljača, M.; Marcelić, Š.; Ban, D.; Grozić, K.; Lukić, M.; Užila, Z.; et al. Determination of the variability of biophenols and mineral nutrients in olive leaves with respect to cultivar, collection period and geographical location for their targeted and well-timed exploitation. Plants 2020, 9, 1667. [Google Scholar] [CrossRef]
- Abdin, M.Z.; Ahmad, A.; Khan, N.; Khan, I.; Jamal, A.; Iqbal, M. Sulphur Interaction with Other Nutrients. In Sulphur in Plants; Springer: Dordrecht, The Netherlands, 2003; pp. 359–374. [Google Scholar] [CrossRef]
- Varényiová, M.; Ducsay, L.; Ryant, P. Sulphur nutrition and its effect on yield and oil content of oilseed rape (Brassica napus L.). Acta Univ. Agric. Silv. Mendel. Brun. 2017, 65, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Zerihun, G.; Girma, A.; Sheleme, B. Rhizobium inoculation and sulphur fertilizer improved yield, nutrients uptake and protein quality of soybean (Glysine max L.) varieties on Nitisols of Assosa area, Western Ethiopia. Afr. J. Plant Sci. 2017, 11, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, I.R.; Paul, K.B.; Eivazi, F.; Bleich, D. Effects of Foliar Fertilization on Yield, Protein, Oil and Elemental Composition of two Soybean Varieties. Commun. Soil Sci. Plant Anal. 1985, 16, 681–692. [Google Scholar] [CrossRef]
- Belikova, H.; Losak, T.; Mezsaros, M.; Conka, P. The effect of foliar fertilization with sulphur on changes in mineral composition of leaves and fruits of Malus × domestica ‘Gloster’. Acta Hortic. 2018, 1217, 417–421. [Google Scholar] [CrossRef]
- Lavee, S.; Wodner, M. Factors affecting the nature of oil accumulation in fruit of olive (Olea europaea L.) cultivars. J. Hortic. Sci. 1991, 66, 583–591. [Google Scholar] [CrossRef]
- Vuletin Selak, G.; Perica, S.; Goreta Ban, S.; Radunic, M.; Poljak, M. Reproductive success after self-pollination and cross-pollination of olive cultivars in Croatia. HortScience 2011, 46, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Blazakis, K.N.; Kosma, M.; Kostelenos, G.; Baldoni, L.; Bufacchi, M.; Kalaitzis, P. Description of olive morphological parameters by using open access software. Plant Methods 2017, 13, 111. [Google Scholar] [CrossRef] [Green Version]
- Calabriso, N.; Scoditti, E.; Pellegrino, M.; Annunziata Carluccio, M. Olive Oil; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780124079427. [Google Scholar]
- Tandon, H.; Messick, D. Practical Sulphur Guide; Sulphur Institute: Washington, DC, USA, 2002. [Google Scholar]
- Chandel, R.; Sudhakar, P.; Singh, K. Response of sulphur nutrition in mustard—A review. Agric. Rev. 2003, 24, 175–182. [Google Scholar]
- Mansoori, I. Response of canola to nitrogen and sulfur fertilizers. J. Agric. Crop. Sci. 2012, 4, 28–33. [Google Scholar]
- Sattar, A.; Cheema, M.A.; Wahid, M.; Saleem, M.; Hassan, M. Interactive effects of sulphur and nitrogen on growth, yield and quality of canola. Crop. Environ. 2011, 2, 32–37. [Google Scholar]
- Raja, A.; Omar Hattab, K.; Gurusamy, L.; Vembu, G.; Suganya, S. Sulphur application on growth and yield and quality of sesame varieties. Int. J. Agric. Res. 2007, 2, 599–606. [Google Scholar]
- Beltrán, G.; Del Río, C.; Sánchez, S.; Martínez, L. Seasonal changes in olive fruit characteristics and oil accumulation during ripening process. J. Sci. Food Agric. 2004, 84, 1783–1790. [Google Scholar] [CrossRef]
- EEC. Characteristics of olive oil and olive-residue oil and the relevant methods of 401 analyses. Regulation EEC/2568/91 and later modifications. Off. J. Eur. Commun. 1991, L24, 1–83. [Google Scholar]
- Covas, M.I.; Ruiz-Gutiérrez, V.; De La Torre, R.; Kafatos, A.; Lamuela-Raventós, R.M.; Osada, J.; Owen, R.W.; Visioli, F. Minor components of olive oil: Evidence to date of health benefits in humans. Nutr. Rev. 2006, 64, S20–S30. [Google Scholar] [CrossRef]
- Valente, S.; Machado, B.; Pinto, D.C.G.A.; Santos, C.; Silva, A.M.S.; Dias, M.C. Modulation of phenolic and lipophilic compounds of olive fruits in response to combined drought and heat. Food Chem. 2020, 329, 127191. [Google Scholar] [CrossRef] [PubMed]
- Lerma-García, M.J.; Ramis-Ramos, G.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Classification of vegetable oils according to their botanical origin using sterol profiles established by direct infusion mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 973–978. [Google Scholar] [CrossRef] [PubMed]





| ‘Istarska Bjelica’ | ‘Leccino’ | |||
|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | |
| Application 1 (50 days from anthesis) | 19 Jul. 2019 | 15 Jul. 2020 | 20 Jul. 2019 | 16 Jul. 2020 |
| Application 2 (80 days from anthesis) | 18 Aug. 2019 | 14 Aug. 2020 | 19 Aug. 2019 | 15 Aug. 2020 |
| Source of Variation | S (g/kg) | N (g/kg) |
|---|---|---|
| Treatment(T) | ||
| 0 mL SN | 2.55 ± 0.11 b | 16.17 ± 0.80 |
| 7.5 mL SN | 2.75 ± 0.10 b | 15.03 ± 1.11 |
| 15 mL SN | 3.00 ± 0.14 a | 14.78 ± 0.82 |
| 22.5 mL SN | 3.01 ± 0.13 a | 15.55 ± 0.88 |
| p-value | *** | n.s. |
| Cultivar (Cv.) | ||
| Istarska bjelica | 2.57 ± 0.05 b | 13.71 ± 0.59 b |
| Leccino | 3.08 ± 1.00 a | 17.06 ± 0.54 a |
| p-value | *** | *** |
| Year (Y) | ||
| 2019 | 2.56 ± 0.06 b | 13.05 ± 0.59 b |
| 2020 | 3.09 ± 0.09 a | 17.72 ± 0.35 a |
| p-value | *** | *** |
| T × Cv. | n.s. | * |
| T × Y | n.s. | n.s. |
| Cv. × Y | *** | n.s. |
| T × Cv. × Y | n.s. | n.s. |
| Source of Variation | Fruit Yield (kg/Tree) | Fruit Mass (g) | Fruit Length (mm) | Fruit Width (mm) | Stone Mass (g) | Stone Length (mm) | Stone Width (mm) | Fruit Length-to-Width Ratio | Stone Length-to-Width Ratio | Pulp Mass (g) | Pulp Percentage (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T) | |||||||||||
| 0 mL SN | 12.76 ± 2.09 b | 2.47 ± 0.02 d | 19.39 ± 0.08 d | 14.77 ± 0.07 d | 0.47 ± 0.01 d | 13.73 ± 0.06 c | 6.92 ± 0.03 d | 1.32 ± 0.00 ab | 1.99 ± 0.01 | 2.00 ± 0.02 d | 80.33 ± 0.23 c |
| 7.5 mL SN | 17.69 ± 2.99 a | 2.78 ± 0.03 c | 20.01 ± 0.08 c | 15.40 ± 0.07 c | 0.50 ± 0.00 c | 14.23 ± 0.24 b | 7.16 ± 0.03 c | 1.30 ± 0.00 c | 2.02 ± 0.05 | 2.28 ± 0.03 c | 81.23 ± 0.23 b |
| 15 mL SN | 15.11 ± 3.01 ab | 3.14 ± 0.03 b | 20.91 ± 0.07 b | 16.02 ± 0.06 b | 0.54 ± 0.01 b | 14.51 ± 0.06 b | 7.33 ± 0.02 b | 1.31 ± 0.00 bc | 1.98 ± 0.01 | 2.60 ± 0.03 b | 82.06 ± 0.22 a |
| 22.5 mL SN | 17.47 ± 2.49 a | 3.35 ± 0.03 a | 21.62 ± 0.08 a | 16.36 ± 0.06 a | 0.59 ± 0.01 a | 15.11 ± 0.06 a | 7.51 ± 0.02 a | 1.33 ± 0.00 a | 2.02 ± 0.01 | 2.76 ± 0.03 a | 81.55 ± 0.21 b |
| p-value | * | *** | *** | *** | *** | *** | *** | *** | n.s. | *** | *** |
| Cultivar (Cv.) | |||||||||||
| Istarska bjelica | 8.51 ± 1.03 b | 3.39 ± 0.02 a | 21.47 ± 0.05 a | 16.91 ± 0.03 a | 0.47 ± 0.00 b | 13.57 ± 0.03 b | 7.10 ± 0.02 b | 1.27 ± 0.00 b | 1.92 ± 0.01 b | 2.92 ± 0.02 a | 85.99 ± 0.08 a |
| Leccino | 23.01 ± 1.65 a | 2.48 ± 0.02 b | 19.50 ± 0.05 b | 14.36 ± 0.03 b | 0.58 ± 0.00 a | 15.22 ± 0.12 a | 7.36 ± 0.02 a | 1.36 ± 0.00 a | 2.09 ± 0.02 a | 1.91 ± 0.01 b | 76.60 ± 0.09 b |
| p-value | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Year (Y) | |||||||||||
| 2019 | 11.04 ± 1.35 b | 3.10 ± 0.02 a | 20.75 ± 0.05 a | 15.90 ± 0.04 a | 0.61 ± 0.00 a | 14.84 ± 0.05 a | 7.55 ± 0.01 a | 1.31 ± 0.00 b | 1.97 ± 0.00 b | 2.50 ± 0.02 a | 79.72 ± 0.14 b |
| 2020 | 20.47 ± 1.98 a | 2.77 ± 0.02 b | 20.22 ± 0.06 b | 15.38 ± 0.05 b | 0.44 ± 0.01 b | 13.95 ± 0.12 b | 6.91 ± 0.02 b | 1.32 ± 0.00 a | 2.04 ± 0.02 a | 2.33 ± 0.02 b | 82.87 ± 0.16 a |
| p-value | *** | *** | *** | *** | *** | *** | *** | * | ** | *** | *** |
| T × Cv. | ** | *** | *** | *** | * | n.s. | *** | *** | n.s. | *** | ** |
| T × Y | * | ** | * | *** | * | n.s. | * | ** | n.s. | *** | *** |
| Cv. × Y | * | *** | *** | *** | *** | ** | ** | *** | * | *** | *** |
| T × Cv. × Y | n.s. | * | n.s. | * | ** | n.s. | *** | n.s. | n.s. | * | n.s. |
| Source of Variation | Oil Fraction in FOP (%) | Oil Extraction Yield (%) | FFA (%) | PV (eq O2/kg) | K232 | K270 | ΔK |
|---|---|---|---|---|---|---|---|
| Treatment (T) | |||||||
| 0 mL SN | 17.20 ± 1.21 b | 11.08 ± 0.92 c | 0.34 ± 0.03 a | 4.69 ± 0.37 b | 2.02 ± 0.04 | 0.19 ± 0.01 b | −0.00 ± 0.00 |
| 7.5 mL SN | 17.75 ± 1.13 ab | 12.40 ± 1.02 ab | 0.32 ± 0.02 a | 4.56 ± 0.46 b | 2.00 ± 0.04 | 0.20 ± 0.00 ab | −0.00 ± 0.00 |
| 15 mL SN | 17.50 ± 0.97 b | 11.95 ± 1.04 bc | 0.28 ± 0.02 b | 4.37 ± 0.45 b | 1.99 ± 0.05 | 0.19 ± 0.01 b | −0.00 ± 0.00 |
| 22.5 mL SN | 18.62 ± 0.96 a | 13.49 ± 1.21 a | 0.30 ± 0.01 ab | 5.51 ± 0.69 a | 2.04 ± 0.04 | 0.20 ± 0.00 a | −0.00 ± 0.00 |
| p-value | ** | *** | ** | *** | n.s. | ** | n.s. |
| Cultivar (Cv.) | |||||||
| Istarska bjelica | 21.71 ± 0.21 a | 15.82 ± 0.39 a | 0.34 ± 0.01 a | 3.58 ± 0.07 b | 2.12 ± 0.01 a | 0.20 ± 0.00 a | −0.01 ± 0.00 a |
| Leccino | 13.82 ± 0.28 b | 8.64 ± 0.38 b | 0.28 ± 0.02 b | 6.07 ± 0.41 a | 1.89 ± 0.03 b | 0.18 ± 0.01 b | −0.00 ± 0.00 b |
| p-value | *** | *** | *** | *** | *** | *** | ** |
| Year (Y) | |||||||
| 2019 | 18.36 ± 0.76 a | 13.63 ± 0.69 a | 0.25 ± 0.01 b | 5.70 ± 0.46 a | 2.09 ± 0.02 a | 0.18 ± 0.01 b | −0.00 ± 0.00 |
| 2020 | 17.18 ± 0.72 b | 10.83 ± 0.72 b | 0.37 ± 0.01 a | 3.94 ± 0.13 b | 1.94 ± 0.03 b | 0.20 ± 0.00 a | −0.00 ± 0.00 |
| p-value | *** | *** | *** | *** | *** | *** | n.s. |
| T × Cv. | * | n.s. | n.s. | *** | n.s. | * | n.s. |
| T × Y | n.s. | ** | *** | n.s. | n.s. | ** | n.s. |
| Cv. × Y | n.s. | n.s. | n.s. | *** | *** | *** | n.s. |
| T × Cv. × Y | n.s. | n.s. | n.s. | n.s. | n.s. | ** | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcelić, Š.; Vidović, N.; Pasković, I.; Lukić, M.; Špika, M.J.; Palčić, I.; Lukić, I.; Petek, M.; Pecina, M.; Herak Ćustić, M.; et al. Combined Sulfur and Nitrogen Foliar Application Increases Extra Virgin Olive Oil Quantity without Affecting Its Nutritional Quality. Horticulturae 2022, 8, 203. https://doi.org/10.3390/horticulturae8030203
Marcelić Š, Vidović N, Pasković I, Lukić M, Špika MJ, Palčić I, Lukić I, Petek M, Pecina M, Herak Ćustić M, et al. Combined Sulfur and Nitrogen Foliar Application Increases Extra Virgin Olive Oil Quantity without Affecting Its Nutritional Quality. Horticulturae. 2022; 8(3):203. https://doi.org/10.3390/horticulturae8030203
Chicago/Turabian StyleMarcelić, Šime, Nikolina Vidović, Igor Pasković, Marina Lukić, Maja Jukić Špika, Igor Palčić, Igor Lukić, Marko Petek, Marija Pecina, Mirjana Herak Ćustić, and et al. 2022. "Combined Sulfur and Nitrogen Foliar Application Increases Extra Virgin Olive Oil Quantity without Affecting Its Nutritional Quality" Horticulturae 8, no. 3: 203. https://doi.org/10.3390/horticulturae8030203
APA StyleMarcelić, Š., Vidović, N., Pasković, I., Lukić, M., Špika, M. J., Palčić, I., Lukić, I., Petek, M., Pecina, M., Herak Ćustić, M., Ban, D., Grozić, K., Polić Pasković, M., Cukrov, M., Pongrac, P., & Goreta Ban, S. (2022). Combined Sulfur and Nitrogen Foliar Application Increases Extra Virgin Olive Oil Quantity without Affecting Its Nutritional Quality. Horticulturae, 8(3), 203. https://doi.org/10.3390/horticulturae8030203