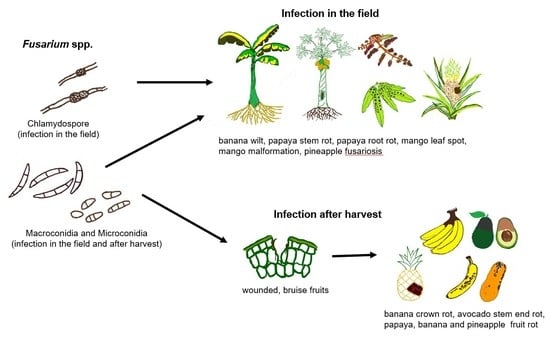
Fusarium Species Associated with Diseases of Major Tropical Fruit Crops
Abstract
:1. Introduction
2. Fusarium Species Associated with Banana Diseases
2.1. Fusarium Wilt
2.2. Crown Rot
2.3. Fruit Rot
3. Fusarium spp. Associated with Papaya Diseases
3.1. Root Rot
3.2. Stem Rot
3.3. Stem End Rot
3.4. Fruit Rot
4. Fusarium Species Associated with Mango Diseases
4.1. Mango Malformation
4.2. Mango Decline and/or Dieback
4.3. Gall
4.4. Leaf Spot
| Disease | Symptoms | Fusarium spp. | Country | References |
|---|---|---|---|---|
| Mango malformation | Vegetative malformation—hypertrophic growth of vegetative buds, swollen axillary buds, and disturbed apical dominance. Growing buds produce distorted shoots bearing small leaves that appear as crowded, unhealthy masses or “witches’ broom” appearance. Malformed seedlings become stunted and eventually die. Floral or inflorescence malformation—inflorescence becomes enlarged, and at the same time, the axes branch abundantly becomes short and thick and produces sterile flowers. Formation of leaves (phyllody) on the inflorescence may occur. | Fusarium moniliforme var. subglutinans | India | [112,113] |
| F. mangiferae | China, Egypt, India, Israel, Malaysia, Oman, South Africa, Spain, Sri Lanka, USA, Australia | [110,116,126] | ||
| F. sterilihyphosum | South Africa, Brazil | [115,117] | ||
| F. mexicanum | Mexico | [121] | ||
| F. tupiense | Brazil, Senegal, Spain | [118,119,120] | ||
| F. pseudocircinatum | Mexico, Dominican Republic | [121,122] | ||
| F. proliferatum | Malaysia, China, Egypt | [123,124,125] | ||
| F. anthophilum, F. fujikuroi, F. incarnatum, F. oxysporum, F. parvisorum, F. scirpi, F. solani, F. verticillioides, three undescribed species (associated with malformation-like symptoms) | Australia | [126] | ||
| F. neocosmosporiellum | Mexico | [127] | ||
| Decline/ Dieback | Uniform pattern of dying back from the crown downwards, accompanied by leaf discoloration. Severe infections cause dying of entire branches, resulting in the death of the infected tree. Other symptoms—include blight, tip dieback, blights, cankers, gummosis, and stem bleeding. | F. solani, F. oxysporum, F. solani | Pakistan | [131,135,136] |
| F. decemcellulare | China | [132] | ||
| Gall | Large galls with a rough and scaly exterior on the main trunks. | F. decemcellulare | Miami, Florida; Mexico; Dominican Republic | [134,139,140] |
| Leaf spot | Discolored lesions or spots on the leaves caused by necrosis of the tissues. | F. proliferatum, F. semitectum, F. chlamydosporum | Malaysia | [144] |
| F. concentricum, F. hainanense, F. mangiferae, F. pernambucanum, F. proliferatum, F. sulawesiense, F. verticillioides | China | [145] |
5. Fusarium spp. Associated with Pineapple Diseases
5.1. Fruitlet Core Rot
5.2. Fusariosis
5.3. Fruit Rot and Leaf Spot
5.4. Heart Rot
5.5. Dieback
| Disease | Symptoms | Fusarium spp. | Country | References |
|---|---|---|---|---|
| Fruitlet core rot | Internal symptoms—brown discoloration in the center of the fruitlet may spread to the fruit core. Infected flesh looks similar to a black spot. External symptoms—dry rot at the infected site, the flesh can remain quite firm, and the fruit remains green. Infected part becomes sunken when the fruits ripen, and the infection is severe. | F. verticillioides | Queensland, Australia | [154] |
| F. guttiforme (formerly F.moniliforme var. subglutinans, F. subglutinans) | Brazil | [168] | ||
| F. ananatum | South Africa, China, Okinawa Prefecture, Japan | [150,156,157] | ||
| F. guttiforme, F. ananatum, F. oxysporum | Paraiba, Pernambuco and Rio Grande do Norte, Brazil | [158] | ||
| F. ananatum, F. oxysporum, F. proliferatum | Reunion Island, France | [159] | ||
| F. proliferatum, F. sacchari, F. oxysporum | Reunion Island, France | [160] | ||
| Fusariosis | Obvious symptoms on affected fruits— discoloration of the infected areas, fruitlets appearing light to dark brown, and rot lesions may spread to the fruit core. Infected areas become sunken, with visible fungal sporulation and gum exudation. Other symptoms—include stunting, chlorosis, shortened stems, bent or dead stems at the apex, and phyllotaxic disruption throughout the plant. | F. moniliforme var. subglutinans (later identified as F. subglutinans) | Cuba | [166,167] |
| F. guttiforme | South and Central America | [168] | ||
| F. ananatum | South Africa | [156] | ||
| F. semitectum, F. fujikuroi | Malaysia | [169,170] | ||
| Fruit Rot and Leaf spot | Leaf spot —leaf discoloration with spot and necrosis | F. oxysporum, F. solani, Fusarium sp., F. proliferatum, F. verticillioides, F. sacchari | Malaysia | [172] |
| Fruit Rot | Fruit rot— rotting of the flesh, formation of brown lesions, and sometimes mycelia appear in the rot lesions. | F. ananatum, F. concentricum, F. fujikuroi, F. guttiforme, F. incarnatum, F. oxysporum, F. polyphialidicum, F. proliferatum, F. temperatum, F. verticillioides | Poland (imported pineapple) | [13] |
| Heart rot | Basal leaf tissue of the youngest leaves (located at the heart of the apical meristem) is affected. Symptoms of soft rot of infected leaves and leaf loss are visible. | Fusarium sp. | South Cotabato and Davao City, the Philippine | [176] |
| Dieback | Drying and yellowing of leaves from the apex (crown) to the base. Diseased plants occurred in patches. | F. oxysporum | Venecia—San Carlos, Costa Rica | [175] |
6. Fusarium Species Associated with Avocado Diseases
6.1. Dieback
6.2. Avocado Wilt
6.3. Post-Harvest Diseases
6.3.1. Stem End Rot
6.3.2. Fruit Rot
| Disease | Symptoms | Fusarium spp. | Country | References |
|---|---|---|---|---|
| Dieback | Wilting and branch dieback due to necrosis of vascular tissue. A white powdery exudate become visible. Infected wood becomes discolored and necrotic. | Fusarium sp. | California, Israel | [181,182,184] |
| F. euwallaceae | California, Israel, Palestine, South Africa | [185,187,188] | ||
| F. obliquiseptatum | Queensland, Australia | [189] | ||
| Wilt | Yellowing of the leaves, loss of vigor, and stunted growth. Wilt progresses, defoliation occurs, and dieback symptoms become visible. Internal symptoms—discoloration of the vascular tissues. | F. oxysporum, F. solani, F. equiseti | Colombia | [191,192] |
| Stem end rot | On ripe fruits, rot lesion emerges as brown to black discoloration at the stem end. As the lesion developed, entire fruit becomes rotten | F. sambucinum, F. solani | South Africa | [200] |
| F. solani, F. equiseti, ‘F. moniliforme’ | Phillipines | [201] | ||
| F. oxysporum | Sri Lanka | [202] | ||
| F. solani, F. oxysporum, F. equiseti | Kenya | [203] | ||
| Fruit rot | Brown, circular spots are visible on the surface of infected fruits. | F. decemcellulare | South Africa | [205] |
| F. crookwellense, F. pallidoroseum (syn. F. semitectum), F. equiseti, F. graminearum | New Zealand | [196] | ||
| F. verticillioides, F. proliferatum | Khon Kaen, Thailand; Port Harcourt, Rivers State, Nigeria | [206,207] |
7. Control Measures
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Martin, F.W. (Ed.) Handbook of Tropical Food Crops; CRC Press: Boca Raton, FL, USA, 1984. [Google Scholar]
- Ploetz, R.C. Fusarium-induced diseases of tropical, perennial crops. Phytopathology 2006, 96, 648–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galán Saúco, V. Potential of minor tropical fruits to become important fruit crops. Acta Hortic. 2013, 975, 581–591. [Google Scholar] [CrossRef]
- Underhill, S.J.R. Fruits of tropical climates, commercial and dietary importance. In Encyclopedia of Food Science and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003. [Google Scholar]
- FAO. Major Tropical Fruits Statistical Compendium 2019; FAO: Rome, Italy, 2020. [Google Scholar]
- Altendorf, S. Major Tropical Fruits Market review 2017; FAO: Rome, Italy, 2019. [Google Scholar]
- Summerell, B.A.; Salleh, B.; Leslie, J.F. A utilitarian approach to Fusarium identification. Plant Dis. 2003, 87, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.N. An overview of ecological and habitat aspects in the genus Fusarium with special emphasis on the soil-borne pathogenic forms. Plant Pathol. Bull. 2007, 16, 97–120. [Google Scholar]
- Lin, Y.H.; Su, C.C.; Chao, C.P.; Chen, C.Y.; Chang, C.J.; Huang, J.W.; Chang, P.F.L. A molecular diagnosis method using real-time PCR for quantification and detection of Fusarium oxysporum f. sp. cubense race 4. Eur. J. Plant Pathol. 2013, 135, 395–405. [Google Scholar] [CrossRef]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O′Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef] [Green Version]
- Bakker, M.G.; Brown, D.W.; Kelly, A.C.; Kim, H.S.; Kurtzman, C.P.; Mccormick, S.P.; O’Donnell, K.L.; Proctor, R.H.; Vaughan, M.M.; Ward, T.J. Fusarium mycotoxins: A trans-disciplinary overview. Can. J. Plant Pathol. 2018, 40, 161–171. [Google Scholar] [CrossRef]
- Ploetz, R.C. (Ed.) Diseases of Tropical Fruit Crops; CABI Publishing: Wallingford, UK, 2003. [Google Scholar]
- Stępień, Ł.; Koczyk, G.; Waśkiewicz, A. Diversity of Fusarium species and mycotoxins contaminating pineapple. J. Appl. Genet. 2013, 54, 367–380. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, K.l.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.; Balajee, S.A.; Schroers, H.J.; Summerbell, R.C.; Robert, V.A.; Crous, P.W.; Zhang, N.; et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef] [Green Version]
- Lassoudière, A. Le bananier et sa Culture; Editions Quae: Versailles, France, 2007. [Google Scholar]
- Heslop-Harrison, J.S.; Schwarzacher, T. Domestication, genomics and the future for banana. Ann. Bot. 2007, 100, 1073–1084. [Google Scholar] [CrossRef] [Green Version]
- FAO. Banana Market Review—Preliminary Results 2020; FAO: Rome, Italy, 2021. [Google Scholar]
- Ploetz, R.C.; Pegg, K.G. Fusarium Wilt. Diseases of Banana, Abaca and Enset; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Stover, R.H. Fusarial Wilt (Panama Disease) of Bananas and Other Musa Species; Commonwealth Mycological Inst.: Kew, UK, 1962. [Google Scholar]
- Ploetz, R.C. Panama disease: Return of the first banana menace. Int. J. Pest Manag. 1994, 40, 326–336. [Google Scholar] [CrossRef]
- Stover, R.H.; Buddenhagen, I.W. Banana breeding: Polyploidy, disease resistance and productivity. Fruits 1986, 41, 175–191. [Google Scholar]
- Ploetz, R.C. Panama disease, an old nemesis rears its ugly head: Part 1. Plant Health Prog. 2005, 6, 8. [Google Scholar] [CrossRef]
- Waite, B.H.; Stover, R.H. Studies on Fusarium wilt of bananas. VI. variability and the cultivars concept in Fusarium oxysporum f. sp cubense. Can. J. Bot. 1960, 38, 985–994. [Google Scholar] [CrossRef]
- Su, H.; Hwang, S.; Ko, W. Fusarial wilt of Cavendish bananas in Taiwan. Plant Dis. 1986, 70, 814–818. [Google Scholar]
- Moore, N.; Pegg, K.G.; Langdon, P.W.; Smith, M.K.; Whiley, A.W. Current research on Fusarium wilt of banana in Australia. In INIBAP/ASPNET Proceedings of the International Symposium on Recent Developments in Banana Cultivation Technology, Pingtung, Taiwan, 14–18 December 1992; Valmayor, R.V., Hwang, S.C., Ploetz, R.C., Lee, S.W., Roa, V.N., Eds.; Taiwan Banana Research Institute: Pingtung, Taiwan, 1993; pp. 270–284. [Google Scholar]
- Buddenhagen, I. Understanding strain diversity in Fusarium oxysporum f. sp. cubense and history of introduction of “Tropical Race 4” to better manage banana production. Acta Hortic. 2009, 828, 193–204. [Google Scholar] [CrossRef]
- Ploetz, R.C. Fusarium Wilt. Handbook of Diseases of Banana, Abaca and Enset; Jones, D.R., Ed.; CABI: Wallingford, UK, 2018. [Google Scholar]
- Viljoen, A.; Mostert, D.; Chiconela, T.; Beukes, I.; Fraser, C.; Dwyer, J.; Murray, H.; Amisse, J.; Matabuana, E.L.; Tazan, G.; et al. Occurrence and spread of the banana fungus Fusarium oxysporum f. sp. cubense TR4 in Mozambique. S. Afr. J. Sci. 2020, 116, 1–11. [Google Scholar]
- Ploetz, R.C. Management of Fusarium wilt of banana: A review with special reference to tropical race 4. Crop Prot. 2015, 73, 7–15. [Google Scholar] [CrossRef]
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The epidemiology of Fusarium Wilt of banana. Front. Plant Sci. 2019, 10, 1395. [Google Scholar] [CrossRef] [Green Version]
- Ploetz, R.C.; Pegg, K.G. Fusarium wilt of banana and Wallace’s line: Was the disease originally restricted to his Indo-Malayan region? Australas. Plant Pathol. 1997, 26, 239–249. [Google Scholar] [CrossRef]
- Ploetz, R. Diseases and pests: A review of their importance and management. Infomusa 2004, 13, 11–16. [Google Scholar]
- Ordoñez, N.; García-Bastidas, F.; Laghari, H.B.; Akkary, M.Y.; Harfouche, E.N.; al Awar, B.N.; Kema, G.H.J. First report of Fusarium oxysporum f. sp. cubense tropical Race 4 causing Panama disease in Cavendish bananas in Pakistan and Lebanon. Plant Dis. 2016, 100, 209. [Google Scholar]
- Bentley, S.; Moore, N.Y.; Pegg, K.G.; Gerlach, K.S.; Smith, L.J. Genetic characterization and detection of Fusarium wilt. In Banana Fusarium Wilt Management: Towards Sustainable Cultivation; Molina, A.B., Masdek, N.H.N., Liew, K.W., Eds.; INIBAP: Los Banños, Philippines, 2001; pp. 143–151. [Google Scholar]
- García-Bastidas, F.; Ordóñez, N.; Konkol, J.; Al-Qasim, M.; Naser, Z.; Abdelwali, M.; Salem, N.; Waalwijk, C.; Ploetz, R.C.; Kema, G.H.J. First Report of Fusarium oxysporum f. sp. cubense Tropical Race 4 associated with Panama disease of banana outside Southeast Asia. Plant Dis. 2014, 98, 694. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, L.; Liang, C.; Wang, G.; Dai, Q.; Huang, J. Differential colonization patterns of bananas (Musa spp.) by physiological race 1 and race 4 isolates of Fusarium oxysporum f. sp. cubense. J. Phytopathol. 2015, 163, 807–817. [Google Scholar] [CrossRef]
- O’Neill, W.T.; Henderson, J.; Pattemore, J.A.; O’Dwyer, C.; Perry, S.; Beasley, D.R.; Tan, Y.P.; Smyth, A.L.; Goosem, C.H.; Thomson, K.M.; et al. Detection of Fusarium oxysporum f. sp. cubense tropical race 4 strain in northern Queensland. Australas. Plant Dis. Notes. 2016, 11, 33. [Google Scholar] [CrossRef] [Green Version]
- Maymon, M.; Shpatz, U.; Harel, Y.M.; Levy, E.; Elkind, G.; Teverovsky, E.; Gofman, R.; Haberman, A.; Zemorski, R.; Ezra, N.; et al. First Report of Fusarium oxysporum f. sp. cubense Tropical race 4 Causing Fusarium Wilt of Cavendish Bananas in Israel. Plant Dis. 2018, 98, 694. [Google Scholar] [CrossRef]
- Chittarath, K.; Mostert, D.; Crew, K.S.; Viljoen, A.; Kong, G.; Molina, A.B.; Thomas, J.E. First report of Fusarium oxysporum f. sp. cubense tropical race 4 (VCG01213/16) associated with Cavendish bananas in Laos. Plant Dis. 2018, 102, 449. [Google Scholar] [CrossRef]
- Zheng, S.J.; García-Bastidas, F.A.; Li, X.; Zeng, L.; Bai, T.; Xu, S.; Yin, K.; Li, H.; Fu, G.; Yu, Y.; et al. New geographical insights of the latest expansion of Fusarium oxysporum f.sp. cubense tropical Race 4 into the greater Mekong subregion. Front. Plant Sci. 2018, 9, 457. [Google Scholar] [CrossRef] [Green Version]
- Hung, T.N.; Hung, N.Q.; Mostert, D.; Viljoen, A.; Chao, C.P.; Molina, A.B. First report of Fusarium Wilt on Cavendish bananas, caused by Fusarium oxysporum f. sp. cubense tropical Race 4 (VCG 01213/16), in Vietnam. Plant Dis. 2018, 102, 448. [Google Scholar] [CrossRef]
- Damodaran, T.; Mishra, V.K.; Jha, S.K.; Gopal, R.; Rajan, S.; Ahmed, I. First report of Fusarium Wilt in banana caused by Fusarium oxysporum f. sp. cubense tropical Race 4 in India. Plant Dis. 2019, 3, 1022. [Google Scholar] [CrossRef]
- García-Bastidas, F.A.; Quintero-Vargas, J.C.; Ayala-Vasquez, M.; Schermer, T.; Seidl, M.F.; Santos-Paiva, M.; Noguera, A.M.; Aguilera-Galvez, C.; Wittenberg, A.; Hofstede, R.; et al. First report of Fusarium Wilt Tropical Race4 in Cavendish bananas caused by Fusarium odoratissimum in Colombia. Plant Dis. 2020, 104, 994. [Google Scholar] [CrossRef]
- Aguayo, J.; Cerf, I.; Folscher, A.B.; Fourrier-Jeandel, C.; Ioos, R.; Matthews, M.C.; Mostert, D.; Renault, C.; Wilson, V.; Viljoen, A. First report of Fusarium oxysporum f. sp. cubense tropical race 4 (TR4) causing banana wilt in the Island of Mayotte. Plant Dis. 2020, 10, 1094. [Google Scholar] [CrossRef]
- Özarslandan, M.; Akgül, D.S. First Report of Fusarium oxysporum f. sp. cubense Race 4 Causing Fusarium Wilt Disease of Banana in Turkey. Plant Dis. 2020, 104, 974. [Google Scholar] [CrossRef]
- Olivares, B.O.; Rey, J.C.; Lobo, D.; Navas-Cortés, J.A.; Gómez, J.A.; Landa, B.B. Fusarium Wilt of Bananas: A Review of Agro-Environmental Factors in the Venezuelan Production System Affecting Its Development. Agronomy 2021, 11, 986. [Google Scholar] [CrossRef]
- Gubbuk, H.; Altinkaya, L.; Balkıç, R. Banana: A very profitable tropical crop for Turkey. Chron. Horticult. 2017, 57, 20–25. [Google Scholar]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium Wilt of Banana: Current Knowledge on Epidemiology and Research Needs Toward Sustainable Disease Management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ploetz, R.C. Fusarium wilt of banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef] [Green Version]
- Maryani, N.; Lombard, L.; Poerba, Y.S.; Subandiyah, S.; Crous, P.W.; Kema, G.H.J. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Stud. Mycol. 2019, 92, 155–194. [Google Scholar] [CrossRef]
- Torres Bedoya, E.; Bebber, D.P.; Studholme, D.J. Taxonomic revision of the banana Fusarium wilt TR4 pathogen is premature. Phytopathology 2021, 111, 2141–2145. [Google Scholar] [CrossRef]
- Krauss, U.; Johanson, A. Recent advances in the control of crown rot of banana in the Windward Islands. Crop Prot. 2000, 19, 151–159. [Google Scholar] [CrossRef]
- Lassois, L.; de Bellaire, D.L. Chapter 3: Crown rot disease of bananas. In Postharvest Decay; Bautista-Baños, S., Ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Finlay, A.R.; Lubin, C.; Brown, A.E. The banana stalk as a source of inoculum of fungal pathogens which cause crown rot. Trop. Sci. 1992, 32, 343–352. [Google Scholar]
- Alvindia, D.; Kobayashi, T.; Tanda, S. Identification of fungi isolated from nonchemical banana fruits and farms in the Philippines. J. Agric. Sci. Tokyo Univ. Agric. 2002, 47, 78–97. [Google Scholar]
- Lassois, L.; Jijakli, M.H.; Chillet, M.; de Lapeyre de Bellaire, L. Crown rot of bananas: Preharvest factors involved in postharvest disease development and integrated control methods. Plant Dis. 2010, 94, 648–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandran, D.R.; Thara Susha, S. Etiology of fungi causing postharvest crown rot of robusta variety banana in Kerala. Trop. Agric. 2021, 59, 124–133. [Google Scholar]
- Anthony, S.; Abeywickrama, K.; Dayananda, R.; Wijeratnam, S.W.; Arambewela, L. Fungal pathogens associated with banana fruit in Sri Lanka, and their treatment with essential oils. Mycopathologia 2004, 157, 91–97. [Google Scholar] [CrossRef]
- de Lapeyre de Bellaire, L.; Chillet, M.; Dubois, C.; Mourichon, X. Importance of different sources of inoculum and dispersal methods of conidia of Colletotrichum musae, the causal agent of banana anthracnose, for fruit contamination. Plant Pathol. 2000, 49, 782–790. [Google Scholar] [CrossRef]
- de Lapeyre de Bellaire, L.; Mourichon, X. The pattern of fungal contamination of the banana bunch during its development and potential influence on incidence of crown-rot and anthracnose diseases. Plant Pathol. 1997, 46, 481–489. [Google Scholar] [CrossRef]
- Shillingford, C.A. Control of banana fruit rots and of fungi that contaminate washing water. Trop. Sci. 1977, 19, 197–203. [Google Scholar]
- Ploetz, R.C.; Thomas, J.E.; Slabaugh, W. Diseases of banana and plantain. In Diseases of Tropical Fruit Crops; Ploetz, R.C., Ed.; CABI Publishing: Wallingford, UK, 2003; pp. 73–134. [Google Scholar]
- Triest, D.; Hendrickx, M. Postharvest disease of banana caused by Fusarium musae: A public health concern? PLoS Pathog. 2016, 12, e1005940. [Google Scholar] [CrossRef] [Green Version]
- Kamel, M.A.M.; Cortesi, P.; Saracchi, M. Etiological agents of crown rot of organic bananas in Dominican Republic. Postharvest Biol. Technol. 2016, 120, 112–120. [Google Scholar] [CrossRef]
- Du, Y.X.; Chen, F.R.; Shi, N.N.; Ruan, H.C. First report of Fusarium chlamydosporum causing banana crown rot in Fujian Province, China. Plant Dis. 2017, 101, 1048. [Google Scholar] [CrossRef]
- Waliullah, S.; Fonsah, E.G.; Brock, J.; Li, Y.; Ali, M.E. First Report of Crown Rot of Banana Caused by Fusarium proliferatum in Georgia, USA. Plant Dis. 2022, 106, 1526. [Google Scholar] [CrossRef] [PubMed]
- Van Hove, F.; Waalwijk, C.; Logrieco, A.; Munaut, F.; Moretti, A. Gibberella musae (Fusarium musae) sp. nov., a recently discovered species from banana is sister to F. verticillioides. Mycologia 2011, 103, 570–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triest, D.; Piérard, D.; De Cremer, K.; Hendrickx, M. Fusarium musae infected banana fruits as potential source of human fusariosis: May occur more frequently than we might think and hypotheses about infection. Commun. Integr. Biol. 2016, 9, e1162934. [Google Scholar] [CrossRef]
- Knight, C. Pathogenicity of some fungi associated with crown rot of bananas. J. Phytopathol. 1982, 104, 13–18. [Google Scholar] [CrossRef]
- Griffee, P.J. Pathogenicity of some fungi isolated from diseased crowns of banana hands. J. Phytopathol. 1976, 85, 206–216. [Google Scholar] [CrossRef]
- Marin, D.H.; Sutton, T.B.; Blankenship, S.M.; Swallow, W.H. Pathogenicity of fungi associated with crown rot of bananas in Latin America on Grande Naine and disease-resistant hybrid bananas. Plant Dis. 1996, 80, 525–528. [Google Scholar] [CrossRef]
- Umaña-Rojas, G.; García, J. Frequency of organisms associated with crown rot of bananas in integrated and organic production systems. Acta Hortic. 2011, 906, 211–217. [Google Scholar] [CrossRef]
- Umana-Rojas, G.; Garcia, J. Pathogenicity of organisms associated with banana crown rot in two banana cultivars. Acta Hortic. 2011, 906, 219–223. [Google Scholar] [CrossRef]
- Hirata, T.; Kimishima, E.; Aoki, T.; Nirenberg, H.I.; O’Donnell, K. Morphological and molecular characterization of Fusarium verticillioides from rotten banana imported into Japan. Mycoscience 2001, 42, 155–166. [Google Scholar] [CrossRef]
- Salem, N.M.; AlMomany, A.M.; Tahat, M.M.; Aldakil, H. First report of Fusarium verticillioides causing banana fruit rot in Jordan. Plant Dis. 2020, 104, 3255. [Google Scholar] [CrossRef]
- Jiménez, M.; Logrieco, A.; Bottalico, A. Occurrence and pathogenicity of Fusarium species in banana fruits. J. Phytopathol. 1993, 137, 214–220. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Bahkali, A.H. Toxigenic profiles and trinucleotide repeat diversity of Fusarium species isolated from banana fruits. Biotechnol. Biotechnol. Equip. 2015, 29, 324–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghuthaymi, M.; Alshehri, W.A.; Al-Maary, K.S.; Bahkali, N.A.; Alkahtani, M.D.F.; Alarfaj, A.A.; AlNadhari, S.; Ameen, F. Mycotoxigenicity of Fusarium isolated from banana fruits: Combining phytopathological assays with toxin concentrations. J. King Saud Univ. Sci. 2020, 32, 1482–1485. [Google Scholar] [CrossRef]
- Abd-Elsalam, K. First report of Fusarium thapsinum on imported banana fruits into Saudi Arabia. Pest Technol. 2009, 3, 25–27. [Google Scholar]
- Molnár, O.; Bartók, T.; Szécsi, Á. Occurrence of Fusarium verticillioides and Fusarium musae on banana fruits marketed in Hungary. Acta Microbiol. Immunol. Hung. 2015, 62, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Latiffah, Z.; Mazzura, W.C.; Heng, K.W.; Baharuddin, S. Fusarium species associated with fruit rot of banana (Musa spp.), papaya (Carica papaya) and guava (Psidium guajava). Malays. J. Microbiol. 2012, 8, 127–130. [Google Scholar]
- Abd Murad, N.B.; Mohamed Nor, N.M.I.; Shohaimi, S.; Mohd Zainudin, N.A.I. Genetic diversity and pathogenicity of Fusarium species associated with fruit rot disease in banana across Peninsular Malaysia. J. Appl. Microbiol. 2017, 123, 1533–1546. [Google Scholar] [CrossRef]
- Supriya, S.; Girisham, S.; Reddy, S.M. Incidence of post-harvest fungal diseases of banana fruit in Warangal market. Indian Phytopathol. 2009, 62, 103–105. [Google Scholar]
- Bashar, M.; Shamsi, S.; Hossain, M. Fungi associated with rotten fruits in Dhaka metropolis. Bangladesh J. Bot. 2012, 41, 115–117. [Google Scholar] [CrossRef]
- Riolo, M.; Aloi, F.; Faedda, R.; Cacciola, S.O.; Pane, A. First Report of Postharvest Fruit Rot Caused by Fusarium sacchari on Lady Finger Banana in Italy. Plant Dis. 2020, 104, 2290. [Google Scholar] [CrossRef] [Green Version]
- Farina, V.; Passafiume, R.; Tinebra, I.; Scuderi, D.; Saletta, F.; Gugliuzza, G.; Gallotta, A.; Sortino, G. Postharvest application of Aloe vera gel-based edible coating to improve the quality and storage stability of fresh-cut papaya. J. Food Qual. 2020, 2020, 8303140. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.K.; Mitra, S.K.; Saran, S. Papaya production in India—History, present status and future prospects. Acta Hort. 2016, 1111, 87–94. [Google Scholar] [CrossRef]
- Singh, S.K.; Kumar, R. Etiology, symptoms and molecular characterization of papaya root rot-a new and serious threat. Indian Phytopathol. 2015, 68, 348–349. [Google Scholar]
- Gupta, A.K.; Choudhary, R.; Bashyal, B.M.; Rawat, K.; Singh, D.; Solanki, I.S. First report of root and stem rot disease on papaya caused by Fusarium falciforme in India. Plant Dis. 2019, 103, 2676. [Google Scholar] [CrossRef]
- Vega-Gutiérrez, T.A.; Tirado-Ramírez, M.A.; López-Urquídez, G.A.; Angulo-Castro, A.; Martínez-Gallardo, J.A.; López-Orona, C.A. Fusarium falciforme (FSSC 3 + 4) causing root and stem rot in papaya (Carica papaya) in Mexico. Plant Dis. 2019, 103, 2681. [Google Scholar] [CrossRef]
- Correia, K.C.; Souza, B.O.; Câmara, M.P.S.; Michereff, S.J. First report of stem rot of papaya caused by Fusarium solani species complex in Brazil. Plant Dis. 2013, 97, 140. [Google Scholar] [CrossRef]
- Hunter, J.E.; Buddenhagen, I.W. Incidence, epidemiology and control of fruit diseases of papaya in Hawaii. Trop. Agric. 1972, 49, 61–72. [Google Scholar]
- Alvarez, A.M.; Nishijima, W.T. Postharvest diseases of papaya. Plant Dis. 1987, 71, 681–686. [Google Scholar] [CrossRef]
- Manalastas, E.T.; Pordesimo, A.N. Fungal flora of ripening papaya [Carica papaya L.] fruits (Philippines). In Proceedings of the 13th Anniversary and Annual Convention of the Pest Control Council of the Philippines, Baguio, Philippines, 5–8 May 1982. [Google Scholar]
- Yaguchi, Y.; Nakamura, S. Stem-end rot of papaya and its pathogens. Jpn. J. Phytopathol. 1992, 58, 30–36. [Google Scholar] [CrossRef]
- Nery-Silva, F.A.; Machado, J.D.; Resende, M.L.; Lima, L.C. Inoculation methodology of papaya fruits with fungi causing stem-end rot. Ciênc. Agrotec. 2007, 31, 1374–1379. [Google Scholar] [CrossRef] [Green Version]
- Coates, L.; Johnson, G. Chapter 33: Postharvest diseases of fruit and vegetables. In Plant Pathogens and Plant Diseases; Brown, J., Ogle, H., Eds.; Rockvale Publications: Cambridge, UK, 1997; pp. 533–547. [Google Scholar]
- Nishijima, W. Fusarium solani: Fruit and Seedling Rot of Papaya. Crop Knowl. Master. 1993. Available online: http://www.extento.hawaii.edu/kbase/crop/Type/f_solan.htm (accessed on 5 March 2021).
- Rahman, M.A.; Mahmud, T.M.M.; Kadir, J.; Abdul, R.R.; Begum, M.M. Major postharvest fungal diseases of Papaya cv. ‘Sekaki’ in Selangor, Malaysia. Pertanika J. Tropi. Agric. Sci. 2008, 31, 27–34. [Google Scholar]
- Sharddha, G.; Lal, A.A. Eco-friendly management of post harvest fungal pathogen causing Fusarium rot of papaya (Carica papaya L.) in Allahabad. Natl. Acad. Sci. Lett. 2010, 33, 227–233. [Google Scholar]
- Margaret, O.; Egwari, L. Fruit, Leaf and Stem Diseases of Carica papaya. J. Int. Sci. Pub. 2015, 3, 398–407. [Google Scholar]
- Pathak, V.N.; Goyal, J.P.; Bhatanagar, L.G. Effect of chemical and hot water treatment on Fusarium and Rhizopus rots of papaya. Indian Phytopathol. 1976, 29, 210–211. [Google Scholar]
- Gupta, A.K.; Pathak, V.N. Epidemiology and management of papaya fruit rots. Summa Phytopathol. 1990, 16, 92–105. [Google Scholar]
- Helal, R.B.; Hosen, S.; Shamsi, S. Mycoflora associated with post-harvest disease of papaya (Carica papaya l.) and their pathogenic potentiality. Bangladesh J. Bot. 2018, 47, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Oke, O.A.; Banjoko, K.M. The effects of Penicillium digitatum and Fusarium oxysporum rots on nutritional content of pawpaw (Carica papaya L.). Mycopathologia 1991, 116, 199–202. [Google Scholar] [CrossRef]
- Vivek, K.; Prasad, B.; Anuradha Sandhya, S.; Sourabh, C.; Sujit, W.; Rupali, C.; Kanade, M.B. Studies on Post-Harvest Fungal Pathogens of Papaya Fruits (Carica papaya). Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2176–2180. [Google Scholar]
- Tharanathan, R.N.; Yashoda, H.M.; Prabha, T.N. Mango (Mangifera indica L.). The king of fruits. Food Rev. Int. 2006, 22, 95–123. [Google Scholar] [CrossRef]
- Saúco, V.G. Trends in world mango production and marketing. Acta Hortic. 2017, 1183, 351–364. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Freeman, S. Foliar, floral and soilborne diseases. In The Mango: Botany, Production and Uses; Litz, R.E., Ed.; CABI: Wallingford, UK, 2009; pp. 231–302. [Google Scholar]
- Freeman, S.; Shtienberg, D.; Maymon, M.; Levin, A.G.; Ploetz, R.C. New insights into mango malformation disease epidemiology lead to a new integrated management strategy for subtropical environments. Plant Dis. 2014, 98, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marasas, W.F.O.; Ploetz, R.C.; Wingfield, M.J.; Wingfield, B.D.; Steenkamp, E.T. Mango malformation disease and the associated Fusarium species. Phytopathology 2006, 96, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Summanwar, A.S.; Raychaudhuri, S.P.; Phatak, S.C. Association of the fungus Fusarium moniliforme Sheld. with the malformation in mango (Mangifera indica L.). Indian Phytopathol. 1966, 19, 227–228. [Google Scholar]
- Varma, A.; Lele, V.C.; Raychaudhuri, S.P.; Ram, A.; Sang, A. Mango malformation: A fungal disease. J. Phytopathol. 1974, 79, 254–257. [Google Scholar] [CrossRef]
- Steenkamp, E.; Britz, H.; Coutinho, T.; Wingfield, B.; Marasas, W.; Wingfield, M. Molecular characterization of Fusarium subglutinans associated with mango malformation. Mol. Plant Pathol. 2000, 1, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Britz, H.; Steenkamp, E.T.; Coutinho, T.A.; Wingfield, B.D.; Marasas, W.F.O.; Wingfield, M.J. Two new species of Fusarium section Liseola associated with mango malformation. Mycologia 2002, 94, 722–730. [Google Scholar] [CrossRef]
- Freeman, S.; Maimon, M.; Pinkas, Y. Use of GUS transformants of Fusarium subglutinans for determining etiology of mango malformation disease. Phytopathology 1999, 89, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Lima, C.S.; Pfenning, L.H.; Costa, S.S.; Campos, M.A.; Leslie, J.F. A new Fusarium lineage within the Gibberella fujikuroi species complex is the main causal agent of mango malformation disease in Brazil. Plant Pathol. 2009, 58, 33–42. [Google Scholar] [CrossRef]
- Lima, C.S.; Pfenning, L.H.; Costa, S.S.; Abreu, L.M.; Leslie, J.F. Fusarium tupiense sp. nov., a member of the Gibberella fujikuroi complex that causes mango malformation in Brazil. Mycologia 2012, 104, 1408–1419. [Google Scholar] [CrossRef]
- Senghor, A.L.; Sharma, K.; Kumar, P.L.; Bandyopadhyay, R. First report of mango malformation disease caused by Fusarium tupiense in Senegal. Plant Dis. 2012, 96, 1582. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Cazorla, F.M.; Hermoso, J.M.; Guirado, E.; Maymon, M.; Toŕes, J.A.; Freeman, S.; de Vicente, A. First report of mango malformation disease caused by Fusarium mangiferae in Spain. Plant Dis. 2012, 96, 286. [Google Scholar] [CrossRef] [PubMed]
- Otero-Colina, G.; Rodríguez-Alvarado, G.; Fernández-Pavía, S.; Maymon, M.; Ploetz, R.C.; Aoki, T.; O’Donnell, K.; Freeman, S. Identification and characterization of a novel etiological agent of mango malformation disease in Mexico. Fusarium mexicanum sp. nov. Phytopathology 2010, 100, 1176–1184. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Lopez, E.L.V.I.S.; Mora-Aguilera, J.A.; Nava-Diaz, C.; Villegas-Monter, A.N.G.E.L.; Tovar-Pedraza, J.M.; Serra, C.; Batista-Marte, C.M. First report of Fusarium pseudocircinatum causing mango malformation disease in Dominican Republic. Plant Dis. 2016, 100, 1501. [Google Scholar] [CrossRef]
- Mohamed Nor, N.M.I.; Salleh, B.; Leslie, J.F. Fusarium species associated with mango malformation in peninsular Malaysia. J. Phytopathol. 2013, 161, 617–624. [Google Scholar] [CrossRef]
- Zhan, R.L.; Yang, S.J.; Ho, H.H.; Liu, F.; Zhao, Y.L.; Chang, J.M.; He, Y.B. Mango malformation disease in South China caused by Fusarium proliferatum. J. Phytopathol. 2010, 158, 721–725. [Google Scholar] [CrossRef]
- Haggag, W.M.; Hazza, M.; Sehab, A.; El-Wahab, M.A. Epidemiology and the association of the Fusarium species with the mango malformation disease in Egypt. Nat. Sci. 2010, 8, 128–135. [Google Scholar]
- Liew, E.C.Y.; Laurence, M.H.; Pearce, C.A.; Shivas, R.G.; Johnson, G.I.; Tan, Y.P.; Edwards, J.; Perry, S.; Cooke, A.W.; Summerell, B.A. Review of Fusarium species isolated in association with mango malformation in Australia. Australas. Plant Pathol. 2016, 45, 547–559. [Google Scholar] [CrossRef]
- Molina-Cárdenas, L.; López-Urquídez, G.A.; Amarillas-Bueno, L.A.; Vega-Gutierrez, T.A.; Tirado-Ramírez, M.A.; Velázquez-Alcaraz, T.D.J.; Velarde-Félix, S.; López-Orona, C.A. Mango malformation disease caused by Fusarium neocosmosporiellum in Mexico. Can. J. Plant Pathol. 2021, 43, 714–721. [Google Scholar] [CrossRef]
- Pernezny, K.; Ploetz, R.C. Some Common Diseases of Mango in Florida. Plant Pathol. Fact Sheet. PP-23. 2000. Available online: http://plantpath.ifas.ufl.edu/takextpub/FactSheets/pp0023.pdf (accessed on 23 May 2022).
- Naqvi, S.A.H.; Perveen, R. Mango quick decline manifestation on various cultivars at plants of particular age in the vicinity of district Multan. Pak. J. Phytopathol. 2015, 27, 31–39. [Google Scholar]
- Kalidindi, U. Mango Decline: Mango Diseases Is a Big Threat to Mango Industry. 2015. Available online: https://www.krishisandesh.com/mango-diseases-a-threat-to-mango-industry (accessed on 10 May 2021).
- Khaskheli, M.I.; Jiskani, M.M.; Soomro, M.H.; Talpur, M.A.; Poussio, G.B. Prevalence of mango sudden decline/death syndrome (msds) on various varieties at the orchards of different age in the vicinity of Tando Qaiser, Syderabad, Sindh. Pak. J. Agric., Agrol. Eng. Vet. Sci. 2011, 27, 160–167. [Google Scholar]
- Qi, Y.; Pu, J.; Zhang, X.; Zhang, H.; Lu, Y.; Yu, Q.; Zhang, H.; Xie, Y. First report of dieback of mango caused by Fusarium decemcellulare in China. J. Phytopathol. 2013, 161, 735–738. [Google Scholar] [CrossRef]
- Al Adawi, A.O.; Deadman, M.L.; Al Rawahi, A.K.; Al Maqbali, Y.M.; Al Jahwari, A.A.; Al Saadi, B.A.; Al Amri, I.S.; Wingfield, M.J. Aetiology and causal agents of mango sudden decline disease in the Sultanate of Oman. Eur. J. Plant Pathol. 2006, 116, 247–254. [Google Scholar] [CrossRef]
- Ploetz, R.C. The major diseases of mango: Strategies and potential for sustainable management. Acta Hortic. 2004, 645, 137–150. [Google Scholar] [CrossRef]
- Abbasi, Q.D.; Jan, N.D.; Mahar, A.N.; Panhwar, A.; Khuhro, R.D. Etiology of mango tree mortality in Pakistan. Int. J. Fruit Sci. 2008, 8, 237–250. [Google Scholar] [CrossRef]
- Mahmood, A.; Gill, M.A. Quick decline of mango and in vitro response of fungicides against the disease. International J. Agric. Biol. 2002, 4, 39–40. [Google Scholar]
- Pathan, M.A.; Nizamani, Z.A.; Jiskani, M.M.; Wagan, K.H. Pathogenicity and control Fusarium equiseti (Corda) Sacc. causing tip dieback disease of mango (Mangifera indica L.). Pak. Agr. J. Agr. Eng. Vet. Sci. 2004, 20, 43–47. [Google Scholar]
- Ploetz, R.C. First Report of Fusarium decemcellulare as a Pathogen of Mango in the United States. Plant Dis. 1996, 80, 1207. [Google Scholar] [CrossRef]
- Angulo, S.M.; Villapudua, J.R. Buba of mango (Mangifera indica L.) in the state of Sinaloa, Mexico. Phytopathology 1982, 72, 171. [Google Scholar]
- García-López, E.; Mora-Aguilera, J.A.; Hernández Castro, E.; Jiménez-Vásquez, C.J.; Batista-Marte, C.M.; Serra, C. First report of gall disease in mango trees caused by Fusarium decemcellulare in Dominican Republic. J. Plant Pathol. 2017, 99, 287–304. [Google Scholar]
- Horst, R. Westcott’s Plant Disease Handbook, 7th ed.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Lucas, G.; Campbell, L. Introduction to Plant Diseases Identification and Management, 2nd ed.; Springer: New York, NY, USA, 1992. [Google Scholar]
- Agrios, G. Plant Pathology, 5th ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Omar, N.H.; Mohd, M.; Mohamed Nor, N.M.I.; Zakaria, L. Characterization and pathogenicity of Fusarium species associated with leaf spot of mango (Mangifera indica L.). Microb. Pathog. 2018, 114, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yu, Z.; Li, Q.; Tang, L.; Guo, T.; Huang, S.; Mo, J.; Hsiang, T.; Luo, S. Fusarium species associated with leaf spots of mango in China. Microb. Pathog. 2021, 150, 104736. [Google Scholar] [CrossRef] [PubMed]
- Morton, J. Fruits of Warm Climates; J.F. Morton: Miami, FL, USA, 1987. [Google Scholar]
- Bartholomew, D.; Paull, R.; Rohrbach, K. The Pineapple Botany, Production, and Uses; CAB International: New York, NY, USA, 2003. [Google Scholar]
- Rohrbach, K.G.; Apt, W.J. Nematode and disease problems of pineapple. Plant Dis. 1986, 70, 81–87. [Google Scholar] [CrossRef]
- Joy, P.P.; Sindhu, G. Diseases of pineapple (Ananas comosus): Pathogen, symptoms, infection, spread & management. Consult. Agosto 2012. pp. 4–5. Available online: https://kau.in/sites/default/files/documents/diseases_of_pineapple.pdf (accessed on 10 August 2022).
- Gu, H.; Zhan, R.-L.; Zhang, L.-B.; Gong, D.-Q.; Jia, Z.-W. First report of Fusarium ananatum causing pineapple fruitlet core rot in China. Plant Dis. 2015, 99, 1653. [Google Scholar] [CrossRef]
- Rohrbach, K.G.; Johnson, M.W. Pests, diseases and weeds. In The Pineapple: Botany, Production and Uses; Bartholomew, D.P., Paull, R.E., Rohrbach, K.G., Eds.; CABI Publishing: Wallingford, UK, 2003; pp. 203–252. [Google Scholar]
- Johnson, M.O. The Pineapple, 1st ed.; Paradise of the Pacific Press: Honolulu, HI, USA, 1935. [Google Scholar]
- Edmonstone-Sammons, C. The Fungal Flora Associated with Black Spot of Pineapples. In Some Aspects of the Microflora of Citrus Soils; Rhodes University: Grahamstown, South Africa, 1955. [Google Scholar]
- Oxenham, B. Etiology of fruitlet core rot of pineapple in Queensland. Qld. J. Agric. Sci. 1962, 19, 27–31. [Google Scholar]
- Petty, G.J.; Tustin, H.A.; Dicks, H.M. Control of black spot disease/fruitlet core rot in queen pineapple with integrated mealybug, pineapple fruit mite and fungus control programmes. Acta Hortic. 2006, 702, 143–149. [Google Scholar] [CrossRef]
- Jacobs, A.; Van Wyk, P.S.; Marasas, W.F.; Wingfield, B.D.; Wingfield, M.J.; Coutinho, T.A. Fusarium ananatum sp. nov. in the Gibberella fujikuroi species complex from pineapples with fruit rot in South Africa. Fungal Biol. 2010, 114, 515–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashiro, M.; Arasaki, C.; Takushi, T.; Ooshiro, A.; Ajitomi, A.; Takeuchi, M.; Moromizato, C.; Aoki, T. Fruitlet core rot of pineapple (Ananas comosus) caused by Fusarium ananatum in Japan. Jpn. J. Phytopathol. 2019, 85, 25–29. [Google Scholar] [CrossRef]
- Souza, W.C.O.; Nascimento, L.C.; Oliveira, M.D.M.; Porcino, M.M.; Silva, H.A.O. Genetic diversity of Fusarium spp. in pineapple ‘Pérola’ cultivar. Eur. J. Plant Pathol. 2018, 150, 853–868. [Google Scholar] [CrossRef]
- Barral, B.; Chillet, M.; Doizy, A.; Grassi, M.; Ragot, L.; Léchaudel, M.; Durand, N.; Rose, L.J.; Viljoen, A.; Schorr-Galindo, S. Diversity and toxigenicity of fungi that cause pineapple fruitlet core rot. Toxins 2020, 12, 339. [Google Scholar] [CrossRef]
- Vignassa, M.; Meile, J.C.; Chiroleu, F.; Soria, C.; Leneveu-Jenvrin, C.; Schorr-Galindo, S.; Chillet, M. Pineapple mycobiome related to fruitlet core rot occurrence and the influence of fungal species dispersion patterns. J. Fungi 2021, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, K.G. Fusariosis. In Compendium of Tropical Diseases; Ploetz, R.C., Zentmeyer, G.A., Nishijima, W.T., Rohrbach, K.G., Ohr, H.D., Eds.; American Phytopathological Society Press: Saint Paul, MN, USA, 1994; pp. 45–56. [Google Scholar]
- Ploetz, R.C. Significant diseases in the tropics that are caused by species of Fusarium. In Fusarium: Paul Nelson Memorial Symposium; Summerell, B.A., Leslie, J.F., Backhouse, D., Bryden, W.L., Burgess, L.W., Eds.; American Phytopathological Society Press: Saint Paul, MN, USA, 2001; pp. 295–309. [Google Scholar]
- Ventura, J.; Maffia, L.; Chaves, G. Field induction of fusariosis in pineapple fruit with Fusarium moniliforme Sheld. var. subglutinans WR. & RG. Fruits 1981, 36, 707–710. [Google Scholar]
- Matos, A.P. Pathological Aspects of the Pineapple Crop with Emphasis on the Fusariose. Rev. Fac. Agron. 1995, 21, 179–197. [Google Scholar]
- Rohrbach, K.G.; Schmitt, D.P. Fusariosis. In Compendium of Tropical Fruit Diseases; Ploetz, R.C., Zentmyer, G.A., Nishijima, W.T., Rohrbach, K.G., Ohr, H.D., Eds.; American Phytopathological Society Press: Saint Paul, MN, USA, 1998; p. 49. [Google Scholar]
- Hidalgo, O.B.; Pires de Matos, A.; Cabral, R.S.; Tussel, R.T.; Arzola, M.; Santos, R.; Pérez, M.C. Phytotoxic effect of culture filtrate from Fusarium subglutinans the causal agent of fusariose of pineapple (Ananas comosus (L.) Merr. Euphytica 1998, 104, 73–77. [Google Scholar] [CrossRef]
- Borras, O.; Santos, R.; Matos, A.P.; Cabral, R.S.; Arzola, M. A first attempt to use a Fusarium subglutinans culture filtrate for the selection of pineapple cultivars resistant to fusariose disease. Plant Breed. 2001, 120, 435–438. [Google Scholar] [CrossRef]
- Nirenberg, H.I.; O’Donnell, K. New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia 1998, 90, 434–458. [Google Scholar] [CrossRef]
- Ibrahim, N.F.; Mohd, M.H.; Mohamed Nor, N.M.I.; Zakaria, L. Fusarium fujikuroi causing fusariosis of pineapple in peninsular Malaysia. Ibrahim, NF. Australas. Plant Dis. Notes. 2016, 11, 21. [Google Scholar] [CrossRef]
- Ibrahim, N.F.; Mohd, M.H.; Mohamed Nor, N.M.I.; Zakaria, L. Pathogenicity of Fusarium semitectum and Fusarium chlamydosporum associated with pineapple fusariosis. Malays. J. Microbiol. 2016, 12, 164–170. [Google Scholar]
- Ibrahim, N.F.; Mohd, M.H.; Nor, N.M.I.M.; Zakaria, L. First report of Fusarium oxysporum and F. solani associated with pineapple rot in peninsular Malaysia. Plant Dis. 2015, 99, 1650. [Google Scholar] [CrossRef]
- Ibrahim, N.F.; Mohd, M.H.; Mohamed Nor, N.M.I.; Zakaria, L. Characterization of Fusarium spp. associated with pineapple fruit rot and leaf spot in Peninsular Malaysia. J. Phytopathol. 2017, 165, 718–726. [Google Scholar] [CrossRef]
- Ibrahim, N.F.; Mohd, M.H.; Mohamed Nor, N.M.I.; Zakaria, L. Mycotoxigenic potential of Fusarium species associated with pineapple diseases. Arch. Phytopathol. Plant Prot. 2020, 53, 217–229. [Google Scholar] [CrossRef]
- Parry, D.W. Plant Pathology in Agriculture; Cambridge University Press: New York, NY, USA, 1990. [Google Scholar]
- Green, J.; Nelson, S. Heart and root rots of pineapples. UH-CTAHR PD-106 2015, 106, 1–7. [Google Scholar]
- Dionio, B.; Bacoba, C.J.; Puig, C.; Ramos, F.R. Fusarium Heart Rot: First Report on pineapple in South Cotabato and Davao City, Philippines. Southeast. Philipp. J. Res. Dev. 2020, 25, 183–196. [Google Scholar] [CrossRef]
- Vásquez Jiménez, J.; Mata Granados, X. Diagnosis of Fusarium oxysporum in the cultivation of pineapple Ananas comosus (L.) Merr. Net. J. Agric. Sci. 2014, 2, 107–112. [Google Scholar]
- Oyedeji, E.; Kareem, K. In-vitro evaluation of some fungicides against Botrydiplodia theobromae: Causal pathogen of pineapple dieback. Am. J. Exp. Agric. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Guzmán, L.F.; Machida-Hirano, R.; Borrayo, E.; Cortés-Cruz, M.; Espíndola-Barquera, M.D.C.; Heredia García, E. Genetic Structure and Selection of a Core Collection for Long Term Conservation of Avocado in Mexico. Front. Plant Sci. 2017, 8, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD-FAO. Agricultural Outlook; FAO: Rome, Italy; OECD Publishing: Paris, France, 2021–2029. [Google Scholar]
- Eskalen, A.; Gonzalez, A.; Wang, D.H.; Twizeyimana, M.; Mayorquin, J.S.; Lynch, S.C. First report of a Fusarium sp. and its vector tea shot hole borer (Euwallacea fornicatus) causing Fusarium dieback on avocado in California. Plant Dis. 2012, 96, 1070. [Google Scholar] [CrossRef]
- Eskalen, A.; Stouthamer, R.; Lynch, S.C.; Rugman-Jones, P.F.; Twizeyimana, M.; Gonzalez, A.; Thibault, T. Host Range of Fusarium Dieback and Its Ambrosia Beetle (Coleoptera: Scolytinae) Vector in Southern California. Plant Dis. 2013, 97, 938–951. [Google Scholar] [CrossRef] [Green Version]
- Mendel, Z.; Protasov, A.; Sharon, M.; Zveibil, A.; Ben Yehuda, S.B.; O’Donnell, K.; Rabaglia, R.; Wysoki, M.; Freeman, S. An Asian ambrosia beetle Euwallacea fornicatus and its novel symbiotic fungus Fusarium sp. pose a serious threat to the Israeli avocado industry. Phytoparasitica 2012, 40, 235–238. [Google Scholar] [CrossRef]
- Freeman, S.; Protasov, A.; Sharon, M.; Mohotti, K.; Eliyahu, M.; Okon-Levy, N.; Maymon, M.; Mendel, Z. Obligate feed requirement of Fusarium sp. nov., an avocado wilting agent, by the ambrosia beetle Euwallacea aff. fornicata. Symbiosis 2013, 58, 245–251. [Google Scholar] [CrossRef]
- Freeman, S.; Sharon, M.; Maymon, M.; Mendel, Z.; Protasov, A.; Aoki, T.; Eskalen, A.; O’Donnell, K. Fusarium euwallaceae sp. nov. a symbiotic fungus of Euwallacea sp., an invasive ambrosia beetle in Israel and California. Mycologia 2013, 105, 1595–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, F.; Carrillo, J.D.; Mayorquin, J.S.; Ndinga-Muniania, C.; Stajich, J.E.; Stouthamer, R.; Huang, Y.T.; Lin, Y.T.; Chen, C.Y.; Eskalen, A. Two Novel Fungal Symbionts Fusarium kuroshium sp. nov. and Graphium kuroshium sp. nov. of Kuroshio Shot Hole Borer (Euwallacea sp. nr. fornicatus) Cause Fusarium Dieback on Woody Host Species in California. Plant Dis. 2018, 102, 1154–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazen, S.; Rola, M.; Ziad, F.; Osama, A.; Khadija, N.; Jehad, R.; Ruba, A. First report of Fusarium euwallaceae on avocado trees in Palestine. Arch. Phytopathol. Plant Prot. 2019, 52, 930–937. [Google Scholar]
- Berg, N.; Toit, M.D.; Morgan, S.W.; Fourie, G.; Beer, Z.W. First report of Fusarium euwallaceae causing necrotic lesions on Persea americana in South Africa. Plant Dis. 2019, 103, 1774. [Google Scholar] [CrossRef]
- Aoki, T.; Smith, J.A.; Kasson, M.T.; Freeman, S.; Geiser, D.M.; Geering, A.D.W.; O’Donnell, K. Three novel Ambrosia Fusarium Clade species producing clavate macroconidia known (F. floridanum and F. obliquiseptatum) or predicted (F. tuaranense) to be farmed by Euwallacea spp. (Coleoptera: Scolytinae) on woody hosts. Mycologia 2019, 111, 919–935. [Google Scholar] [CrossRef]
- Ramírez-Gil, J.G.; Morales-Osorio, J. Diseases and disorders associated with different stages of crop development and factors that determine the incidence in Hass avocado crops. Rev. Cer. 2021, 68, 71–82. [Google Scholar] [CrossRef]
- Ramirez Gil, J.G. Avocado wilt complex disease, implications and management in Colombia. Rev. Fac. Nac. Agron. Medellin. 2018, 71, 8525–8541. [Google Scholar] [CrossRef]
- Ramírez-Gil, J.G.; Gilchrist Ramelli, E.; Morales Osorio, J.G. Economic impact of the avocado (cv. Hass) wilt disease complex in Antioquia, Colombia, crops under different technological management levels. Crop Prot. 2017, 101, 103–115. [Google Scholar] [CrossRef]
- Fuentes-Aragón, D.; Silva-Rojas, H.V.; Guarnaccia, V.; Mora-Aguilera, J.A.; Aranda-Ocampo, S.; Bautista-Martínez, N.; Téliz-Ortíz, D. Colletotrichum species causing anthracnose on avocado fruit in Mexico: Current status. Plant Pathol. 2020, 69, 1513–1528. [Google Scholar] [CrossRef]
- Hofer, K.M.; Braithwaite, M.; Braithwaite, L.J.; Sorensen, S.; Siebert, B.; Pather, V.; Goudie, L.; Williamson, L.; Alexander, B.J.R.; Toome-Heller, M. First report of Colletotrichum fructicola, C. perseae, and C. siamense causing anthracnose disease of avocado (Persea americana) in New Zealand. Plant Dis. 2021, 105, 1564. [Google Scholar] [CrossRef]
- Galsurker, O.; Diskin, S.; Maurer, D.; Feygenberg, O.; Alkan, N. Fruit stem-end rot. Horticulturae 2018, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- Hartill, W.F.T.; Everett, K.R. Inoculum sources and infection pathways of pathogens causing stem-end rots of ‘Hass’ avocado (Persea americana). N. Z. J. Crop Hortic. Sci. 2002, 30, 249–260. [Google Scholar] [CrossRef]
- Menge, J.A.; Ploetz, R.C. Diseases of avocado. In Diseases of Tropical Fruit Crops; Ploetz, R.C., Ed.; CABI Publishing: Wallingford, UK, 2003; pp. 35–71. [Google Scholar]
- Twizeyimana, M.; Förster, H.; McDonald, V.; Wang, D.H.; Adaskaveg, J.E.; Eskalen, A. Identification and pathogenicity of fungal pathogens associated with stem-end rot of avocado in California. Plant Dis. 2013, 97, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, V.; Vitale, A.; Cirvilleri, G.; Aiello, D.; Susca, A.; Epifani, F.; Perrone, G.; Polizzi, G. Characterisation and pathogenicity of fungal species associated with branch cankers and stem-end rot of avocado in Italy. Eur. J. Plant Pathol. 2016, 146, 963–976. [Google Scholar] [CrossRef]
- Darvas, J.M.; Kotze, J.M.; Wehnef, F.C. Pathogenicity of fungi causing pre and post-harvest diseases of avocado fruit. Phytophylactica 1987, 19, 489–493. [Google Scholar]
- Suratos, S.C.M. Interaction of molds associated with stem-end rot in avocado (Persea Americana Mill.) fruit. CLSU Sci. J. 2005, 25, 63–64. [Google Scholar]
- Nilmini, R.K.; Panapitiya, D.; Abeywickrama, K.; Kuruppu, M. Morphological and molecular identification of fungal species associated with postharvest stem-end rot disease of avocado in Sri Lanka. Sri Lanka J. Food Agric. 2020, 6, 47–56. [Google Scholar] [CrossRef]
- Wanjiku, E.K.; Waceke, J.W.; Wanjala, B.W.; Mbaka, J.N. Identification and pathogenicity of fungal pathogens associated with stem end rots of avocado fruits in Kenya. Int. J. Microbiol. 2020, 2020, 4063697. [Google Scholar] [CrossRef]
- Yahia, E.M. Avocado. In Crop Post-Harvest: Science and Technology, Perishables; Rees, D., Farrell, G., Orchard, J., Eds.; Wiley-Blackwell: Oxford, UK, 2012; pp. 159–179. [Google Scholar]
- Darvas, J.M.; Kotze, J.M. Post-harvest diseases of avocados. S. Afr. Avocado Grow. Assoc. Yearb. 1981, 4, 63–66. [Google Scholar]
- Jitjak, W.; Sanoamuang, N. Application of cost-effective coating materials supplemented with different types of local essential oil to control Fusarium verticillioides (Sacc.) Nerenberg from post-harvest avocado′ fruits. Int. J. Agric. Tech. 2021, 17, 883–898. [Google Scholar]
- Iyanyi, N.G.; Ataga, A.E.; Rotimi, I.S.; Blessing, I. Molecular identification of fungi associated with avocado (Persea americana Mill.) fruits. Agro-Science 2021, 20, 80–86. [Google Scholar] [CrossRef]
- Nel, B.; Steinberg, C.; Labuschagne, N.; Viljoen, A. Evaluation of fungicides and sterilants for potential application in the management of Fusarium wilt of banana. Crop Prot. 2007, 26, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Jamil, F.N.; Tang, C.N.; Saidi, N.B.; Lai, K.S.; Baharum, N.A. Fusarium wilt in banana: Epidemics and management strategies. In Horticultural Crops; Kossi Baimey, H., Hamamouch, N., Adjiguita Kolombia, Y., Eds.; IntechOpen: London, UK, 2019; pp. 229–331. [Google Scholar] [CrossRef] [Green Version]
- Zorrilla-Fontanesi, Y.; Pauwels, L.; Panis, B.; Signorelli, S.; Vanderschuren, H.; Swennen, R. Strategies to revise agrosystems and breeding to control Fusarium wilt of banana. Nat. Food 2020, 1, 599–604. [Google Scholar] [CrossRef]
- Ismaila, A.A.; Ahmad, K.; Siddique, Y.; Wahab, M.A.A.; Kutawa, A.B.; Abdullahi, A.; Zobir, S.A.M.; Abdu, A.; Abdullah, S.N.A. Fusarium Wilt of Banana: Current update and sustainable disease control using classical and essential oils approaches. Hortic. Plant J. 2023, 9, 1–28. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for management of soilborne diseases in crop production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Eskalen, A.; Faber, B.A. Agriculture: Avocado Pest Management Guidelines. UC IPM Pest Management Guidelines: Avocado. 2016. UC ANR Publication 3436. Available online: https://ipm.ucanr.edu/agriculture/avocado/fusarium-dieback (accessed on 20 January 2023).
- Duguid, A. Mango Common Dieback The Northern Territory (NT) Rural Review. 2021. Available online: https://industry.nt.gov.au/publications/primary-industry-publications/newsletters/regional-newsletters/rural-review/nt-rural-review-november-2021/mango-common-dieback (accessed on 20 January 2023).
- Shukla, P.K.; Fatima, T. Dieback: The great constraint in perennial fruit crops. In Precision Agriculture and Sustainable Crop Production; Chourasia, H.K., Acharya, K., Singh, V.K., Eds.; Today & Tomorrow’s Printers and Publishers: New Delhi, India, 2020; pp. 197–211. [Google Scholar]
- Eckert, J.W. Recent developments in the chemical control of postharvest diseases. Acta Hortic. 1990, 269, 477–494. [Google Scholar] [CrossRef]
- González-Estrada, R.; Blancas-Benítez, F.M.; Velázquez-Estrada, R.; Montaño-Leyva, B.; Ramos-Guerrero, A.; Aguirre-Güitrón, L.; Moreno-Hernández, C.; Coronado-Partida, L.; Herrera-González, J.A.; Rodríguez-Guzmán, C.A.; et al. Alternative Eco-Friendly Methods in the Control of Post-Harvest Decay of Tropical and Subtropical Fruits. In Modern Fruit Industry; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Martinez, P.; Ledezma-Morales, A.; Romero-Islas, L.D.C.; Ramos-Guerrero, A.; Romero-Islas, J.; Rodríguez-Pereida, C.; Casas-Junco, P.; Coronado-Partida, L.; González-Estrada, R.R. Antifungal activity of chitosan against postharvest fungi of tropical and subtropical fruits. In Chitin-Chitosan-Myriad Functionalities in Science and Technology; InTech: London, UK, 2018; pp. 311–322. [Google Scholar] [CrossRef] [Green Version]
- Mejdoub-Trabelsi, B.; Touihri, S.; Ammar, N.; Riahi, A.; Daami-Remadi, M. Effect of chitosan for the control of potato diseases caused by Fusarium species. J. Phytopathol. 2020, 168, 18–27. [Google Scholar] [CrossRef]
- Mohd Israfi, N.A.; Mohd Ali, M.I.A.; Manickam, S.; Sun, X.; Goh, B.H.; Tang, S.Y.; Ismail, N.; Abdull Razis, A.F.; Ch′ng, S.E.; Chan, K.W. Essential oils and plant extracts for tropical fruits protection: From farm to table. Front. Plant Sci. 2022, 13, 9992703. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–286. [Google Scholar] [CrossRef]
- Williamson, S.M.; Guzman, M.; Marin, D.H.; Ana, O.; Jin, X.; Sutton, T.B. Evaluation of Pseudomonas syringae strain ESC-11 for biocontrol of crown rot and anthracnose of banana. Biol. Control 2008, 46, 279–286. [Google Scholar] [CrossRef]
- Govender, V.; Korsten, L. Evaluation of different formulations of Bacillus blicheniformis in mango pack house trials. Biol. Control 2006, 37, 237–242. [Google Scholar] [CrossRef]
- Lurie, S. Postharvest heat treatments. Postharvest Biol.Technol. 1998, 14, 257–269. [Google Scholar] [CrossRef]
- Dissanayake, P.K. Postharvest Heat Treatments to Extend the Shelf Life of Banana (Musa spp.) Fruits. Adv. Trends Agric. Sci. 2019, 1, 27–37. [Google Scholar]
- Hofman, P.J.; Stubbings, B.; Adkins, M.F.; Meiburg, G.F.; Woolf, A.B. Hot water treatments improve ‘Hass’ avocado fruit quality after cold disinfestation. Postharvest Biol.Technol. 2002, 24, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Couey, H.M. Comparison of hot-water spray and immersion treatments for control of postharvest decay of papaya. Plant Dis. 1984, 68, 436–437. [Google Scholar] [CrossRef]
- Schirra, M.; D’Hallewin, G.; Ben-Yehoshua, S.; Fallik, E. Host-pathogen interactions modulated by heat treatment. Postharvest Biol. Technol. 2000, 21, 71–85. [Google Scholar] [CrossRef]
- Vilaplana, R.; Rosero, A.; Valencia-Chamorro, S. Hot water treatments to control internal rot of ‘MD-2’ pineapple fruit caused by Fusarium verticillioides. Acta Hortic. 2019, 1239, 85–90. [Google Scholar] [CrossRef]
- Summerell, B.A.; Laurence, M.H.; Liew, E.C.; Leslie, J.F. Biogeography and phylogeography of Fusarium: A review. Fungal Divers. 2010, 44, 3–13. [Google Scholar] [CrossRef]
- Burgess, L.W.; Bryden, W. Fusarium: A ubiquitous fungus of global significance. Microbiol. Aust. 2012, 33, 22–25. [Google Scholar] [CrossRef]
- Nucci, M. Epidemiology of Fusarium, a significant emerging group of human opportunistic infections. Int. J. Infect. Dis. 2018, 73, 52. [Google Scholar] [CrossRef]
- Meza-Menchaca, T.; Singh, R.K.; Quiroz-Chávez, J.; García-Pérez, L.M.; Rodríguez-Mora, N.; Soto-Luna, M.; Gastélum-Contreras, G.; Vanzzini-Zago, V.; Sharma, L.; Quiroz-Figueroa, F.R. First demonstration of clinical Fusarium strains causing cross-kingdom infections from humans to plants. Microorganisms 2020, 8, 947. [Google Scholar] [CrossRef] [PubMed]


| Disease | Symptoms | Fusarium spp. | Country | References |
|---|---|---|---|---|
| Fusarium wilt | Leaf-yellowing and discoloration of vascular tissues are typical symptoms. Internal symptoms begin at the site of infection (feeder roots), of which the xylem becomes reddish brown and discolored. The disease develops to the rhizome and the pseudostem. Mature leaves turn yellow and wilted, the disease then progresses to the younger leaves that surround the pseudostem. | F. oxysporum f.sp. cubense TR4 | Northern Territory and Queensland, Australia | [34] |
| Southeast Asia (Malaysia, Indonesia, the Philippines) | [26] | |||
| Jordan | [35] | |||
| China | [36] | |||
| Pakistan and Lebanon | [33] | |||
| Queensland, Australia | [37] | |||
| Taiwan | [27] | |||
| Israel | [38] | |||
| Laos | [39] | |||
| Myanmar | [40] | |||
| Vietnam | [41] | |||
| India | [42] | |||
| Colombia | [43] | |||
| Island of Mayotte | [44] | |||
| Turkey | [45] | |||
| Venezuela, Peru | [46] | |||
| Crown rot | Wounds are the entry point and often occur during harvest. Infected tissues become soft and black before withering, at which point the rot lesion progresses into the fruit pulp. | F. semitectum, F. graminearum | Caribbean | [70] |
| F. oxysporum, F. verticillioides, F. graminearium | Windward Islands | [69] | ||
| F. verticillioides, F. semitectum | Costa Rica | [71] | ||
| F.equiseti, F. incarnatum, F. oxysporum, F. solani, F. verticillioides | the Philippine | [55] | ||
| F. camptoceras, F. oncentricum, F. musarum, F. proliferatum, F. semitectum (syn. F. pallidoroseum), F. subglutinans sensu lato | Not mentioned | [62] | ||
| F. proliferatum, F. semitectum, F. graminearum, F.verticillioides, F. sacchari, F. subglutinans, F.verticillioides | Costa Rica | [72] | ||
| F. incarnatum-equiseti species complex, F. verticillioides, F. sacchari, F. proliferatum, F. solani | Dominican Republic | [64] | ||
| F. chlamydosporum | Fujian Province, China | [65] | ||
| Fruit rot | Fruit rot appears as black or dark brown rot lesions that form on the fruit surface. Smaller lesions may merge and form larger ones. Mycelia develop within the lesion as rot lesions enlarge and can cause extensive rotting. | F. acuminatum, F. camptoceras, F. dimerum, F. equiseti, F. graminearum, F. moniliforme, F. oxysporum, F. proliferatum, F. semitectum var. majus, F. solani, F. subglutinans, Fusarium sp. | Spain and Italy (imported banana) | [76] |
| F. semitectum, F. proliferatum, F. circinatum, F. chlamydosporum, F. solani, F. oxysporum, F. thapsinum | Saudi Arabia (imported banana) | [77,78] | ||
| F. verticillioides, F. musae | Hungary (imported banana) | [80] | ||
| F. oxysporum | Andhra Pradesh, Dhaka, India | [83,84] | ||
| F. verticillioides, F. semitectum, F. solani | Malaysia | [81] | ||
| F. incarnatum, F. equiseti, F. camptoceras, F. solani, F.concolor, F.oxysporum, F. proliferatum, F. verticillioides, F. sacchari, F. concentricum, F. fujikuroi | Malaysia | [82] |
| Disease | Symptoms | Fusarium spp. | Country | References |
|---|---|---|---|---|
| Root rot | Reddish-dark coloration on infected root and stem rot as well as rotting and wilting of the young papaya plant. | F. solani | India | [88] |
| F. falciforme | India, Mexico | [89,90] | ||
| Stem rot | Young papaya plants are commonly infected. The infected stem becomes tender, and black or brown lesions then develop. Rotting roots often progress to the trunk. Other noticeable symptoms include leaf drooping and yellowing. | F. falciforme | India; Mexico | [89,90] |
| F. solani species—complex | Brazil | [91] | ||
| Stem end rot | Symptoms begin with slight browning at the peduncle. Rotting appears after a few days, and the peduncle area becomes blackened and soft. | F. solani | Philippines, Japan, Brazil, Hawaii, India, and the Philippine | [94,95,96,98] |
| Fruit rot | Symptoms emerge as rounded, tender areas that later become small depressions. As these lesions develop, rot and mycelia appear on the surface of the infected fruits | F. acuminatum | India | [102] |
| F. equiseti | India | [103] | ||
| F. solani | Allahabad, India; Malaysia; Nigeria | [81,99,100,101] | ||
| F. semitectum, (syn. F. incarnatum) | Malaysia | [81] | ||
| F. nivale | Bangladesh | [104] | ||
| F. oxysporum | Nigeria | [101,104] | ||
| F. thapsinum, F. clamydosporum | India | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, L. Fusarium Species Associated with Diseases of Major Tropical Fruit Crops. Horticulturae 2023, 9, 322. https://doi.org/10.3390/horticulturae9030322
Zakaria L. Fusarium Species Associated with Diseases of Major Tropical Fruit Crops. Horticulturae. 2023; 9(3):322. https://doi.org/10.3390/horticulturae9030322
Chicago/Turabian StyleZakaria, Latiffah. 2023. "Fusarium Species Associated with Diseases of Major Tropical Fruit Crops" Horticulturae 9, no. 3: 322. https://doi.org/10.3390/horticulturae9030322
APA StyleZakaria, L. (2023). Fusarium Species Associated with Diseases of Major Tropical Fruit Crops. Horticulturae, 9(3), 322. https://doi.org/10.3390/horticulturae9030322