Effects of Ultrasonication and Modified Atmosphere Packaging on the Physicochemical Characteristics and Quality of Ready-to-Eat Pomegranate Arils
Abstract
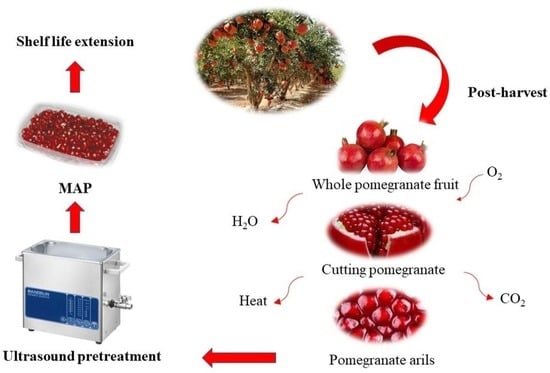
:1. Introduction
2. Materials and Methods
2.1. Weight Loss
2.2. Decay Rate and Microbial Load
2.3. Color Characteristics (L, a*, b*)
2.4. Total Soluble Solids (TSS)
2.5. pH and Titratable Acidity (TA)
2.6. Total Phenols Content (TPC)
2.7. Total Anthocyanin Content (TAC)
2.8. Antioxidant Activity (AA)
2.9. Organoleptic Characteristics
2.10. Statistical Analysis
3. Result and Discussion
3.1. Weight Loss
3.2. Decay Rate and Microbial Load
3.3. Color Characteristics (L, a*, b*)
3.4. Total Soluble Solids (TSS)
3.5. pH and Titratable Acidity (TA)
3.6. Total Phenols Content (TPC)
3.7. Total Anthocyanin Content (TAC)
3.8. Antioxidant Activity (AA)
3.9. Organoleptic Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erkan, M.; Dogan, A. Harvesting of horticultural commodities. In Postharvest Technology of Perishable Horticultural Commodities; Woodhead Publishing: Cambridge, UK, 2019; pp. 129–159. [Google Scholar] [CrossRef]
- Kushwaha, S.C.; Bera, M.B.; Kumar, P. Pomegranate. In Antioxidants in Fruits: Properties and Health Benefits; Springer: Berlin/Heidelberg, Germany, 2020; pp. 295–316. [Google Scholar] [CrossRef]
- Chaves, F.M.; Pavan, I.C.B.; da Silva, L.G.S.; de Freitas, L.B.; Rostagno, M.A.; Antunes, A.E.C.; Bezerra, R.M.N.; Simabuco, F.M. Pomegranate Juice and Peel Extracts are Able to Inhibit Proliferation, Migration and Colony Formation of Prostate Cancer Cell Lines and Modulate the Akt/mTOR/S6K Signaling Pathway. Plant Foods Hum. Nutr. 2020, 75, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Michicotl-Meneses, M.M.; Thompson-Bonilla, M.d.R.; Reyes-López, C.A.; García-Pérez, B.E.; López-Tenorio, I.I.; Ordaz-Pichardo, C.; Jaramillo-Flores, M.E. Inflammation Markers in Adipose Tissue and Cardiovascular Risk Reduction by Pomegranate Juice in Obesity Induced by a Hypercaloric Diet in Wistar Rats. Nutrients 2021, 13, 2577. [Google Scholar] [CrossRef] [PubMed]
- Montero-Calderón, M.; Martín-Belloso, O.; Soliva-Fortuny, R. Fresh-cut fruits: Pineapple. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Academic Press: Cambridge, MA, USA, 2020; pp. 511–518. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Sepulcre, F.; Pujolà, M. Aloe vera based edible coatings improve the quality of minimally processed ‘Hayward’ kiwifruit. Postharvest Biol. Technol. 2013, 81, 29–36. [Google Scholar] [CrossRef]
- Kuwar, U.; Sharma, S.; Tadapaneni, V.R.R. Aloe Vera Gel and Honey-Based Edible Coatings Combined with Chemical Dip as a Safe Means for Quality Maintenance and Shelf Life Extension of Fresh-Cut Papaya. J. Food Qual. 2015, 38, 347–358. [Google Scholar] [CrossRef]
- Belay, Z.A.; Caleb, O.J.; Opara, U.L. Impacts of low and super-atmospheric oxygen concentrations on quality attributes, phytonutrient content and volatile compounds of minimally processed pomegranate arils (cv. Wonderful). Postharvest Biol. Technol. 2017, 124, 119–127. [Google Scholar] [CrossRef]
- Ashrafi, A.; Jokar, M.; Nafchi, A.M. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef]
- Caleb, O.J.; Opara, U.L.; Witthuhn, C.R. Modified Atmosphere Packaging of Pomegranate Fruit and Arils: A Review. Food Bioprocess Technol. 2012, 5, 15–30. [Google Scholar] [CrossRef]
- Banda, K.; Caleb, O.J.; Jacobs, K.; Opara, U.L. Effect of active-modified atmosphere packaging on the respiration rate and quality of pomegranate arils (cv. Wonderful). Postharvest Biol. Technol. 2015, 109, 97–105. [Google Scholar] [CrossRef]
- Wei, S.; Mei, J.; Xie, J. Effects of Different Carbon Dioxide-Modified Atmosphere Packaging and Low-Temperature Storage at 13 °C on the Quality and Metabolism in Mango (Mangifera indica L.). Agriculture 2021, 11, 636. [Google Scholar] [CrossRef]
- Thewes, F.R.; Brackmann, A.; Both, V.; de Oliveira Anese, R.; Schultz, E.E.; Ludwig, V.; Thewes, F.R. Dynamic con-trolled atmosphere based on carbon dioxide production (DCA–CD): Lower oxygen limit establishment, metabolism and overall quality of apples after long-term storage. Postharvest Biol. Technol. 2020, 168, 11128. [Google Scholar] [CrossRef]
- Ramesh, M.; Narendra, G.; Sasikanth, S. A review on biodegradable packaging materials in extending the shelf life and quality of fresh fruits. In Waste Management as Economic Industry Towards Circular Economy; Springer: Berlin/Heidelberg, Germany, 2020; pp. 59–74. [Google Scholar] [CrossRef]
- Floros, J.D.; Matsos, K.I. Introduction to modified atmosphere packaging. In Innovations in Food Packaging; Academic Press: Cambridge, MA, USA, 2005; pp. 159–172. [Google Scholar] [CrossRef]
- Padmanaban, G.; Singaravelu, K.; Annavi, S.T. Increasing the shelf- life of papaya through vacuum packing. J. Food Sci. Technol. 2014, 51, 163–167. [Google Scholar] [CrossRef]
- Kader, A.A. Impact of nut postharvest handling, de-shelling, drying and storage on quality. In Improving the Safety and Quality of Nuts; Woodhead Publishing: Cambridge, UK, 2013; pp. 22–34. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Khayyat, M.; Ranjbari, F.; Maraki, Z. Physiological and quality responses of Shishe-Kab pomegranates to short-term high CO2 treatment and modified atmosphere packaging. Int. J. Fruit Sci. 2018, 18, 287–299. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Ansarifar, E.; Moghaddam, M.M. Extending the shelf life and maintaining quality of minimally-processed pomegranate arils using ascorbic acid coating and modified atmosphere packaging. J. Food Meas. Charact. 2020, 14, 3445–3454. [Google Scholar] [CrossRef]
- Rokalla, P.; Inbaraj, B.S.; Dikkala, P.K.; Sridhar, K.; Dasi, D.S.; Koka, L.; Munakala, R.; Galipothula, R.; Chelli, K.S.R.; Kalletlapally, N.K. Active-Modified Atmosphere Packaging of Ready-to-Eat Pomegranate (Punica granatum L.) Arils at Ambient Temperature for Extending Shelf-Life. Agriculture 2022, 12, 155. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Khayyat, M.; Ranjbari, F.; Maraki, Z. Vacuum packaging optimises quality and reduces postharvest losses of pomegranate fruits. Special Issue-Postharvest Losses. J. Hortic. Postharvest Res. 2019, 2, 15–26. [Google Scholar]
- EL-Eryan, E.E. Influence of different modified atmosphere packaging on quality characteristics of Wonderful pomegranate arils. J. Plant Prod. 2020, 11, 675–680. [Google Scholar] [CrossRef]
- Cho, B.K. Ultrasonic technology. In Nondestructive Evaluation of Food Quality: Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2010; pp. 213–234. [Google Scholar] [CrossRef]
- Andrews, D.R. Fundamentals of Ultrasonics; Cambridge Ultrasonics: Cambridge, UK, 2011. [Google Scholar]
- Aslam, R.; Alam, M.S.; Kaur, J.; Panayampadan, A.S.; Dar, O.I.; Kothakota, A.; Pandiselvam, R. Understanding the effects of ultrasound processng on texture and rheological properties of food. J. Texture Stud. 2022, 53, 775–799. [Google Scholar] [CrossRef]
- Zhi, H.; Liu, Q.; Xu, J.; Dong, Y.; Liu, M.; Zong, W. Ultrasound enhances calcium absorption of jujube fruit by regulating the cellular calcium distribution and metabolism of cell wall polysaccharides. J. Sci. Food Agric. 2017, 97, 5202–5210. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Xiao, Z.; Fu, L. Effect of ultrasonic treatment combined with 1-methylcyclopropene (1-MCP) on storage quality and ethylene receptors gene expression in harvested apple fruit. J. Food Biochem. 2019, 43, e12967. [Google Scholar] [CrossRef]
- Vivek, K.; Subbarao, K.; Srivastava, B. Optimization of postharvest ultrasonic treatment of kiwifruit using RSM. Ultrason. Sonochem. 2016, 32, 328–335. [Google Scholar] [CrossRef]
- Nicolau-Lapeña, I.; Lafarga, T.; Viñas, I.; Abadias, M.; Bobo, G.; Aguiló-Aguayo, I. Ultrasound processing alone or in combination with other chemical or physical treatments as a safety and quality preservation strategy of fresh and processed fruits and vegetables: A review. Food Bioprocess Technol. 2019, 12, 1452–1471. [Google Scholar] [CrossRef]
- Amiri, A.; Ramezanian, A.; Mortazavi, S.M.H.; Hosseini, S.M.H. Ultrasonic potential in maintaining the quality and reducing the microbial load of minimally processed pomegranate. Ultrason. Sonochem. 2021, 70, 105302. [Google Scholar] [CrossRef] [PubMed]
- Ansarifar, E.; Moradinezhad, F. Encapsulation of thyme essential oil using electrospun zein fiber for strawberry preservation. Chem. Biol. Technol. Agric. 2022, 9, 2. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A. Color measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Sadler, G.D.; Murphy, P.A. pH and titratable acidity. In Food Analysis; Springer: Boston, MA, USA, 2010; pp. 219–238. [Google Scholar] [CrossRef]
- Carroll, N.V.; Longley, R.W.; Roe, J.H. The Determination of Glycogen in Liver and Muscle by Use of Anthrone Reagent. J. Biol. Chem. 1956, 220, 583–593. [Google Scholar] [CrossRef]
- Chuah, A.M.; Lee, Y.-C.; Yamaguchi, T.; Takamura, H.; Yin, L.-J.; Matoba, T. Effect of cooking on the antioxidant properties of coloured peppers. Food Chem. 2008, 111, 20–28. [Google Scholar] [CrossRef]
- Swain, T. Analytical methods for flavonoids. In The Chemistry and Biochemistry of Plant Pigments; Goodwin, T.W., Ed.; Academic Press: London, UK, 1965; pp. 543–544. [Google Scholar]
- Sgroppo, S.; Cano, M.P.; de Ancos, B.; Plaza, L. Possible nutritional and health-related value promotion in orange juice preserved by high-pressure treatment. J. Sci. Food Agric. 2002, 82, 790–796. [Google Scholar] [CrossRef]
- Panou, A.A.; Karabagias, I.K.; Riganakos, K.A. The effect of different gaseous ozone treatments on physicochemical characteristics and shelf life of apricots stored under refrigeration. J. Food Process. Preserv. 2018, 42, e13614. [Google Scholar] [CrossRef]
- Hashemi, S.M.B. Effect of pulsed ultrasound treatment compared to continuous mode on microbiological and quality of Mirabelle plum during postharvest storage. Int. J. Food Sci. Technol. 2018, 53, 564–570. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Khayyat, M.; Saeb, H. Combination effects of postharvest treatments and modified atmosphere packaging on shelf life and quality of Iranian pomegranate fruit cv. Sheshi-kab. Int. J. Postharvest Technol. Innov. 2013, 3, 244–256. [Google Scholar] [CrossRef]
- Mphahlele, R.; Fawole, O.A.; Opara, U.L. Influence of packaging system and long term storage on physiological attributes, biochemical quality, volatile composition and antioxidant properties of pomegranate fruit. Sci. Hortic. 2016, 211, 140–151. [Google Scholar] [CrossRef]
- Shaarawi, S.A.; Nagy, K.S. Effect of modified atmosphere packaging on fruit quality of “Wonderful” pomegranate under cold storage conditions. Middle East J. Agric. Res. 2017, 6, 495–505. [Google Scholar]
- Ling, C.; Xu, J.; Shao, S.; Wang, L.; Jin, P.; Zheng, Y. Effect of Ultrasonic Treatment Combined with Peracetic Acid Treatment Reduces Decay and Maintains Quality in Loquat Fruit. J. Food Qual. 2018, 2018, 7564056. [Google Scholar] [CrossRef]
- Rabbani, M.; Victory, M.; Diamond, E. The effect of oral coating and vacuum packing on chemical, microbial and sensory properties of ready-to-eat pomegranate seeds. J. Biomed. Eng. Iran 2016, 4, 633–642. [Google Scholar]
- Dong, X.; He, Y.; Yuan, C.; Cheng, X.; Li, G.; Shan, Y.; Zhu, X. Controlled Atmosphere Improves the Quality, Antioxidant Activity and Phenolic Content of Yellow Peach during the Shelf Life. Antioxidants 2022, 11, 2278. [Google Scholar] [CrossRef]
- Buccheri, M.; Picchi, V.; Grassi, M.; Gandin, D.; Bianchi, G.; Scalzo, R.L. Dynamic changes of antioxidants and fermentative metabolites in apple peel in relation to storage, controlled atmosphere, and initial low oxygen stress. Sci. Hortic. 2021, 288, 110312. [Google Scholar] [CrossRef]
- Teixeira, G.H.D.A.; Santos, L.O.; Júnior, L.C.C.; Durigan, J.F. Effect of carbon dioxide (CO2) and oxygen (O2) levels on quality of ‘Palmer’ mangoes under controlled atmosphere storage. J. Food Sci. Technol. 2018, 55, 145–156. [Google Scholar] [CrossRef]
- Herceg, Z.; Lelas, V.; Jambrak, A.R.; Vukušić, T.; Levaj, B. Influence of thermo-sonication on microbiological safety, color and anthocyanins content of strawberry juice. J. Hyg. Eng. Des. 2013, 4, 26–37. [Google Scholar]
- Ayhan, Z.; Eştürk, O. Overall Quality and Shelf Life of Minimally Processed and Modified Atmosphere Packaged “Ready-to-Eat” Pomegranate Arils. J. Food Sci. 2009, 74, 399–405. [Google Scholar] [CrossRef]
- Tinebra, I.; Sortino, G.; Inglese, P.; Fretto, S.; Farina, V. Effect of Different Modified Atmosphere Packaging on the Quality of Mulberry Fruit (Morus alba L. cv Kokuso 21). Int. J. Food Sci. 2021, 2021, 8844502. [Google Scholar] [CrossRef]
- Palma, A.; Schirra, M.; D’Aquino, S.; La Malfa, S.; Continella, G. Chemical properties changes in pomegranate seeds packaged in polypropylene trays. 1st International Symposium on Pomegranate and Minor Mediterranean Fruits. Acta Hortic. 2006, 818, 323–330. [Google Scholar] [CrossRef]
- Alighourchi, H.R.; Barzegar, M.; Sahari, M.A.; Abbasi, S. Effect of sonication on anthocyanins, total phenolic content, and antioxidant capacity of pomegranate juices. Int. Food Res. J. 2013, 20, 1703–1709. [Google Scholar]
- Cardozo, C.J.M.; Valenzuela, J.R.C. Physico-Chemical Properties of the Soursop Fruit (Annona muricata L. cv. Elita) in Postharvest; American Society of Agricultural and Biological Engineers: Dallas, TX, USA, 2012; p. 1. [Google Scholar] [CrossRef]
- McKenzie, M.J.; Greer, L.A.; Heyes, J.A.; Hurst, P.L. Sugar metabolism and compartmentation in asparagus and broccoli during controlled atmosphere storage. Postharvest Biol. Technol. 2004, 32, 45–56. [Google Scholar] [CrossRef]
- Selcuk, N.; Erkan, M. Changes in phenolic compounds and antioxidant activity of sour–sweet pomegranates cv. ‘Hicaznar’ during long-term storage under modified atmosphere packaging. Postharvest Biol. Technol. 2015, 109, 30–39. [Google Scholar] [CrossRef]
- Cao, S.; Hu, Z.; Pang, B. Optimization of postharvest ultrasonic treatment of strawberry fruit. Postharvest Biol. Technol. 2010, 55, 150–153. [Google Scholar] [CrossRef]
- Brackmann, A.; Anese, R.D.O.; Thewes, F.R.; Fronza, D.; Hamann, J.J. Storability of ‘Tupy’ and ‘Guarani’ blackberries in controlled atmosphere. Bragantia 2016, 75, 240–246. [Google Scholar] [CrossRef]
- Mohideen, F.W.; Solval, K.M.; Li, J.; Zhang, J.; Chouljenko, A.; Chotiko, A.; Prudente, A.D.; Bankston, J.D.; Sathivel, S. Effect of continuous ultra-sonication on microbial counts and physico-chemical properties of blueberry (Vaccinium corymbosum) juice. LWT Food Sci. Technol. 2014, 60, 563–570. [Google Scholar] [CrossRef]
- Zafra-Rojas, Q.Y.; Cruz-Cansino, N.; Ramírez-Moreno, E.; Delgado-Olivares, L.; Villanueva-Sánchez, J.; Alanís-García, E. Effects of ultrasound treatment in purple cactus pear (Opuntia ficus-indica) juice. Ultrason. Sonochem. 2013, 20, 1283–1288. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Hu, B.; Hashim, M.M.; Wu, T.; Lei, S.; Khan, M.A.; Zeng, X. Thermosonication as a potential quality enhancement technique of apple juice. Ultrason. Sonochem. 2014, 21, 984–990. [Google Scholar] [CrossRef]
- Bhat, R.; Kamaruddin, N.S.B.C.; Min-Tze, L.; Karim, A. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason. Sonochem. 2011, 18, 1295–1300. [Google Scholar] [CrossRef]
- Pourcel, L.; Routaboul, J.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef]
- Dziedzic, E.; Błaszczyk, J.; Bieniasz, M.; Dziadek, K.; Kopeć, A. Effect of modified (MAP) and controlled atmosphere (CA) storage on the quality and bioactive compounds of blue honeysuckle fruits (Lonicera caerulea L.). Sci. Hortic. 2020, 265, 109226. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Mason, T.J. Ultrasound processing of fluid foods. In Novel Thermal and Non-Thermal Technologies for Fluid Foods; Academic Press: Cambridge, MA, USA, 2012; pp. 135–165. [Google Scholar] [CrossRef]
- Ochoa, S.; Durango-Zuleta, M.M.; Osorio-Tobón, J.F. Techno-economic evaluation of the extraction of anthocyanins from purple yam (Dioscorea alata) using ultrasound-assisted extraction and conventional extraction processes. Food Bioprod. Process. 2020, 122, 111–123. [Google Scholar] [CrossRef]
- Da Rocha, C.B.; Noreña CP, Z. Microwave-assisted extraction and ultrasound-assisted extraction of bioactive compounds from grape pomace. Int. J. Food Eng. 2020, 16, 20190191. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Golmohamadi, A.; Möller, G.; Powers, J.; Nindo, C. Effect of ultrasound frequency on antioxidant activity, total phenolic and anthocyanin content of red raspberry puree. Ultrason. Sonochem. 2013, 20, 1316–1323. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D.; Soto, E.R.; Barba, F.J. Ultrasound-Assisted Lactic Acid Fermentation of Bakraei (Citrus reticulata cv. Bakraei) Juice: Physicochemical and Bioactive Properties. Fermentation 2023, 9, 37. [Google Scholar] [CrossRef]
- Huang, D.; Men, K.; Li, D.; Wen, T.; Gong, Z.; Sunden, B.; Wu, Z. Application of ultrasound technology in the drying of food products. Ultrason. Sonochem. 2022, 63, 104950. [Google Scholar] [CrossRef]





| Pretreatments | Weight Loss (%) | Decay Rate (%) |
|---|---|---|
| Control (without US and packaging) | 2.02 ± 0.3 a | 24.00 ± 2.0 a |
| 5 min US—VP | 1.39 ± 0.2 c | 12.11 ± 1.7 cd |
| 5 min US—Passive MAP | 1.78 ± 0.2 ab | 14.33 ± 1.2 bc |
| 10 min US—VP | 1.47 ± 0.1 bc | 10.77 ± 0.8 de |
| 10 min US—Passive MAP | 1.80 ± 0.3 a | 14.11 ± 1.4 bc |
| 15 min US—VP | 1.36 ± 0.2 c | 8.11 ± 0.7 e |
| 15 min US—Passive MAP | 1.81 ± 0.3 a | 15.55 ± 1.3 b |
| Storage time | ||
| First day | 0.0 c | 0.0 c |
| 7th day | 1.67 ± 0.2 b | 2.04 ± 0.3 b |
| 14th day | 3.32 ± 0.4 a | 40.38 ± 4.6 a |
| Pretreatments | L Value | a* Index | b* Index |
|---|---|---|---|
| Control (without US and packaging) | 7.04 ± 0.4 ab | 19.80 ± 1.6 c | 1.50 ± 0.09 b |
| 5 min US-VP | 7.28 ± 0.5 a | 22.35 ± 1.9 a | 1.59 ± 0.07 ab |
| 5 min US–Passive MAP | 6.15 ± 0.4 ab | 20.88 ± 1.4 bc | 1.56 ± 0.08 ab |
| 10 min US-VP | 6.75 ± 0.6 ab | 21.62 ± 1.7 ab | 1.64 ± 0.06 a |
| 10 min US–Passive MAP | 6.53 ± 0.5 ab | 20.94 ± 1.8 bc | 1.57 ± 0.09 ab |
| 15 min US-VP | 6.97 ± 0.7 ab | 22.47 ± 2.1 a | 1.60 ± 0.05 ab |
| 15 min US–Passive MAP | 5.74 ± 0.8 b | 21.27 ± 1.8 ab | 1.48 ± 0.07 b |
| Storage time | |||
| 1st day | - | 23.13 ± 1.7 a | 1.82 ± 0.08 a |
| 7th day | - | 21.18 ± 1.9 b | 1.51 ± 0.07 b |
| 14th day | - | 19.69 ± 2.2 c | 1.35 ± 0.06 c |
| Pretreatments | TSS (%) | TA (%) | pH |
|---|---|---|---|
| Control (without US and packaging) | 15.30 ± 1.7 c | 1.61 ± 0.08 b | - |
| 5 min US-VP | 15.83 ± 1.8 ab | 2.11 ± 0.10 a | - |
| 5 min US–Passive MAP | 15.31 ± 1.7 c | 2.01 ± 0.09 a | - |
| 10 min US-VP | 16.01 ± 1.5 a | 2.10 ± 0.10 a | - |
| 10 min US–Passive MAP | 15.35 ± 1.9 c | 2.03 ± 0.08 a | - |
| 15 min US-VP | 15.50 ± 1.6 bc | 2.12 ± 0.07 a | - |
| 15 min US–Passive MAP | 15.37 ± 2.1 c | 2.00 ± 0.11 a | - |
| Storage time | |||
| 1st day | 15.35 ± 1.5 b | 2.34 ± 0.09 a | 3.65 ± 0.2 a |
| 7th day | 15.86 ± 1.7 a | 1.96 ± 0.07 b | 3.33 ± 0.3 b |
| 14th day | 15.36 ± 1.3 b | 1.68 ± 0.07 c | 3.07 ± 0.4 c |
| Pretreatments | TPC (mg 100 g−1 F.W.) | TAC (mg 100 g−1 F.W.) | AA (%) | Taste Index |
|---|---|---|---|---|
| Control (without US and packaging) | 110.98 ± 6.7 b | 140.00 ± 11.6 c | 60.99 ± 5.4 d | 3.00 ± 0.4 c |
| 5 min US-VP | 127.78 ± 8.7 a | 148.01 ± 12.7 a | 63.44 ± 5.7 a | 4.11 ± 0.4 ab |
| 5 min US–Passive MAP | 127.19 ± 7.2 a | 141.29 ± 10.7 bc | 61.64 ± 6.7 cd | 3.88 ± 0.5 ab |
| 10 min US-VP | 128.00 ± 9.4 a | 146.60 ± 9.7 a | 63.95 ± 7.1 a | 4.33 ± 0.6 a |
| 10 min US–Passive MAP | 127.19 ± 6.5 a | 139.78 ± 11.2 c | 62.36 ± 5.8 b | 3.66 ± 0.3 b |
| 15 min US-VP | 127.76 ± 10.2 a | 142.80 ± 10.7 b | 64.02 ± 7.4 a | 4.11 ± 0.5 ab |
| 15 min US–Passive MAP | 127.30 ± 9.1 a | 142.83 ± 12.8 b | 62.21 ± 4.9 bc | 3.55 ± 0.7 bc |
| Storage time | ||||
| 1st day | 137.41 ± 10.5 a | 151.33 ± 11.5 a | 65.16 ± 7.7 b | 5.00 ± 0.6 a |
| 7th day | 129.56 ± 8.7 b | 150.81 ± 8.7 a | 70.39 ± 6.8 a | 4.00 ± 0.4 b |
| 14th day | 108.55 ± 9.3 c | 126.99 ± 10.1 b | 52.43 ± 6.4 c | 2.42 ± 0.3 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradinezhad, F.; Heydari, A.; Ansarifar, E. Effects of Ultrasonication and Modified Atmosphere Packaging on the Physicochemical Characteristics and Quality of Ready-to-Eat Pomegranate Arils. Horticulturae 2023, 9, 809. https://doi.org/10.3390/horticulturae9070809
Moradinezhad F, Heydari A, Ansarifar E. Effects of Ultrasonication and Modified Atmosphere Packaging on the Physicochemical Characteristics and Quality of Ready-to-Eat Pomegranate Arils. Horticulturae. 2023; 9(7):809. https://doi.org/10.3390/horticulturae9070809
Chicago/Turabian StyleMoradinezhad, Farid, Asma Heydari, and Elham Ansarifar. 2023. "Effects of Ultrasonication and Modified Atmosphere Packaging on the Physicochemical Characteristics and Quality of Ready-to-Eat Pomegranate Arils" Horticulturae 9, no. 7: 809. https://doi.org/10.3390/horticulturae9070809
APA StyleMoradinezhad, F., Heydari, A., & Ansarifar, E. (2023). Effects of Ultrasonication and Modified Atmosphere Packaging on the Physicochemical Characteristics and Quality of Ready-to-Eat Pomegranate Arils. Horticulturae, 9(7), 809. https://doi.org/10.3390/horticulturae9070809