Magnetic Characterization of Chromium Intermediates in the Reduction of Chromium (VI) by Glutathione in Acidic Solutions
Abstract
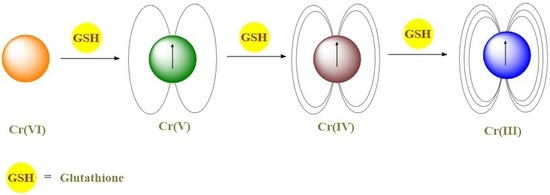
:1. Introduction
2. Materials and Methods
3. Results
[Products] = [Cr(VI)]o{1 − [k1/(k2 − k1)](e−k1t − e−k2t) − e−k1t}.
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC). Chromium, nickel and welding. In Monographs on the Evaluation of Carcinogenic Risks in Humans; IARC: Lyon, France, 1990; Volume 49, pp. 1–648. [Google Scholar]
- Bose, R.N.; Fonkeng, B.S.; Moghaddas, S.; Stroup, D. Mechanisms of DNA damage by chromium(V) carcinogens. Nucleic Acids Res. 1998, 26, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.N.; Moghaddas, S.; Mazzer, P.A.; Dudones, L.P.; Joudah, L.; Stroup, D. Oxidative damage of DNA by chromium(V) complexes: Relative importance of base versus sugar oxidation. Nucleic Acids Res. 1999, 27, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Joudah, L.; Moghaddas, S.; Bose, R.N. DNA oxidation by peroxo-chromium(V) species: Oxidation of guanosine to guanidinohydantoin. Chem. Commun. 2002, 16, 1742–1743. [Google Scholar] [CrossRef]
- Hai, L.; Youngde, L.; Xianglin, S.; Yan, M.; Nar, S.D. Chromium(IV)-Mediated Fenton-like Reaction Causes DNA Damage: Implication to Genotoxicity of Chromate. Ann. Clin. Lab. Sci. 1996, 26, 185–191. [Google Scholar]
- Hai, L.; Youngde, L.; Yan, M.; Xianglin, S.; Nar, S.D. Role of Chromium(IV) in the Chromium(VI)-Related Free Radical Formation, dG Hydroxylation, and DNA Damage. J. Inorg. Biochem. 1996, 64, 25–35. [Google Scholar]
- Casadevall, M.; Da Cruz Fresco, P.; Kortemkamp, A. Chromium(VI)-Mediated DNA damage: Oxidative pathways resulting in the formation of DNA breaks and abasic sites. Chem.-Biol. Interact. 1999, 123, 117–132. [Google Scholar] [CrossRef]
- Slade, P.G.; Hailer, M.K.; Martin, B.D.; Sugden, K.D. Guanine-Specific Oxidation of Double-Stranded DNA by Cr(VI) and Ascorbic Acid Forms Spiroiminodihydantoin and 8-Oxo-2′-deoxyguanosine. Chem. Res. Toxicol. 2005, 18, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.N.; Moghaddas, S.; Gelerinter, E. Long-Lived Chromium(IV) and Chromium(V) Metabolites in the Chromium(VI)-Glutathione Reaction: NMR, ESR, HPLC, and Kinetic Characterization. Inorg. Chem. 1992, 31, 1987–1994. [Google Scholar] [CrossRef]
- Stearns, D.M.; Wetterhahn, K.E. Reaction of Chromium(VI) with Ascorbate Produces Chromium(V), Chromium(IV), and Carbon-Based Radicals. Chem. Res. Toxicol. 1994, 7, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.; Chiu, N.; Shi, X.; Beaubier, J.; Dalal, N. Activation of a Procarcinogen by Reduction: Cr(VI)-Cr(V)-Cr(IV)-Cr(III) A Case Study by Electron Spin Resonance (ESR/PMR). J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 1999, 16, 135–148. [Google Scholar] [CrossRef]
- Chiu, A.; Shi, J.; Lee, W.K.P.; Hill, R.; Wakeman, T.P.; Katz, A.; Xu, B.; Dalal, N.S.; Robertson, J.D.; Chen, C.; et al. Review of Chromium (VI) Apoptosis, Cell-Cycle-Arrest, and Carcinogenesis. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2010, 28, 188–230. [Google Scholar] [CrossRef] [PubMed]
- Connett, P.H.; Wetterhahn, K.E. Metabolism of the Carcinogen Chromate by Cellular Constituents. Struct. Bond. 1983, 54, 93–124. [Google Scholar]
- Macfie, A.; Hagan, E.; Zhitkovich, A. Mechanism of DNA−Protein Cross-Linking by Chromium. Chem. Res. Toxicol. 2010, 23, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.N.; Fonkeng, B.; Barr-David, G.; Farrell, R.P.; Judd, R.J.; Lay, P.A.; Sangster, D.F. Redox Potentials of Chromium(V)/(IV), -(V)/(III), and -(IV)/(III) Complexes with 2-Ethyl-2-hydroxybutanoato(2-/1-) Ligands. J. Am. Chem. Soc. 1996, 118, 7139–7144. [Google Scholar] [CrossRef]
- Marin, R.; Ahuja, Y.; Jackson, G.P.; Laskay, U.; Bose, R.N. Potentially Deadly Carcinogenic Chromium Redox Cycle Involving Peroxochromium(IV) and Glutathione. J. Am. Chem. Soc. 2010, 132, 10617–10619. [Google Scholar] [CrossRef] [PubMed]
- McAuley, A.; Olatunji, M.A. Metal-ion oxidations in solutions. Part XVIII. Characterization, rates and mechanism of formation of the intermediates in the oxidation of thiols by chromium(VI). Can. J. Chem. 1977, 55, 3328–3334. [Google Scholar] [CrossRef]
- Connett, P.H.; Wetterhahn, K.E. In Vitro Reaction of the Carcinogen Chromate with Cellular Thiols and Carboxylic Acids. J. Am. Chem. Soc. 1985, 107, 4282–4288. [Google Scholar] [CrossRef]
- O’Brien, P.; Wang, G.; Wyatt, P.B. Studies of the Kinetics of the Reduction of Chromate by Glutathione and Related Thiols. Polyhedron 1992, 11, 3211–3216. [Google Scholar] [CrossRef]
- Kwong, D.W.J.; Pennington, D.E. Stoichiometry, Kinetics, and Mechanisms of the Chromium(VI) Oxidation of L-Cysteine at Neutral pH. Inorg. Chem. 1984, 23, 2528–2532. [Google Scholar] [CrossRef]
- Lay, P.A.; Levina, A. Kinetics and Mechanism of Chromium(VI) Reduction to Chromium(III) by L-Cysteine in Neutral Aqueous Solutions. Inorg. Chem. 1996, 35, 7709–7717. [Google Scholar] [CrossRef]
- Moghaddas, S.; Gelerinter, E.; Bose, R.N. Mechanisms of Formation and Decomposition of Hypervalent Chromium Metabolites in the Glutathione-Chromium (VI) Reaction. J. Inorg. Biochem. 1995, 57, 135–146. [Google Scholar] [CrossRef]
- Bose, R.N.; Li, D.; Moghaddas, S. Kinetic Method Based on Nuclear Magnetic Resonance Measurements. Anal. Chem. 1991, 63, 2757–2762. [Google Scholar] [CrossRef]
- Warren, H.E. Spin Paramagnetism of Cr+++, Fe+++, and Gd+++ at Liquid Helium Temperatures and in Strong Magnetic Fields. Phys. Rev. 1952, 88, 559–562. [Google Scholar]
- Day, E.P.; Kent, T.A.; Lindahl, P.A.; Munck, E. Squid Measurement of Metalloprotein Magnetization. Biophys. J. 1987, 52, 837–853. [Google Scholar] [CrossRef]
- Levina, A.; Lay, P.A. Solution Structures of Chromium(VI) Complexes with Glutathione and Model Thiols. Inorg. Chem. 2004, 43, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.J.; Shi, X.; Dalal, N.S. Synthesis of Cr(IV)-GSH, Its Identification and Its Free Hydroxyl Radical Generation: A Model Compound for Cr(VI) Carcinogenicity. Biochem. Biophys. Res. Commun. 1997, 235, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Ramsery, C.M.; Dalal, N.S. Crystalline and water soluble Cr(4+) and Cr(5+) model compounds for chromium toxicity studies. Mol. Cell. Biochem. 2004, 255, 113–118. [Google Scholar] [CrossRef]




| [GSH]/[Cr(VI)] | 15 | |
| Ttop/s | 200 | |
| [Cr(VI)]o/mM | 1.02 | Mole Fraction |
| [Cr(VI)]top/mM | 0.128 | 0.12 |
| [Intermediate]top/mM | 0.74 | 0.72 |
| [Products]top/mM | 0.155 | 0.15 |
| [Cr(VI)]/[GSH] = 15 | |
| Intermediate is only Cr(IV) | M = fIntermediate MCr(IV) + fProducts MCr(III) |
| M = 0.72 × 2 μB + 0.15 × 3 μB = 1.9 μB | |
| Intermediate is only Cr(V) | M = fIntermediate MCr(V) + fProducts MCr(III) |
| M = 0.72 × 1 μB + 0.15 × 3 μB = 1.2 μB |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín, R.A.; Bose, R.; Dabrowski, B.; Kolesnik, S. Magnetic Characterization of Chromium Intermediates in the Reduction of Chromium (VI) by Glutathione in Acidic Solutions. Magnetochemistry 2018, 4, 23. https://doi.org/10.3390/magnetochemistry4020023
Marín RA, Bose R, Dabrowski B, Kolesnik S. Magnetic Characterization of Chromium Intermediates in the Reduction of Chromium (VI) by Glutathione in Acidic Solutions. Magnetochemistry. 2018; 4(2):23. https://doi.org/10.3390/magnetochemistry4020023
Chicago/Turabian StyleMarín, Roberto A., Rathindra Bose, Bogdan Dabrowski, and Stanislaw Kolesnik. 2018. "Magnetic Characterization of Chromium Intermediates in the Reduction of Chromium (VI) by Glutathione in Acidic Solutions" Magnetochemistry 4, no. 2: 23. https://doi.org/10.3390/magnetochemistry4020023
APA StyleMarín, R. A., Bose, R., Dabrowski, B., & Kolesnik, S. (2018). Magnetic Characterization of Chromium Intermediates in the Reduction of Chromium (VI) by Glutathione in Acidic Solutions. Magnetochemistry, 4(2), 23. https://doi.org/10.3390/magnetochemistry4020023