Spin Cross-Over (SCO) Complex Based on Unsymmetrical Functionalized Triazacyclononane Ligand: Structural Characterization and Magnetic Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Description of the Structure
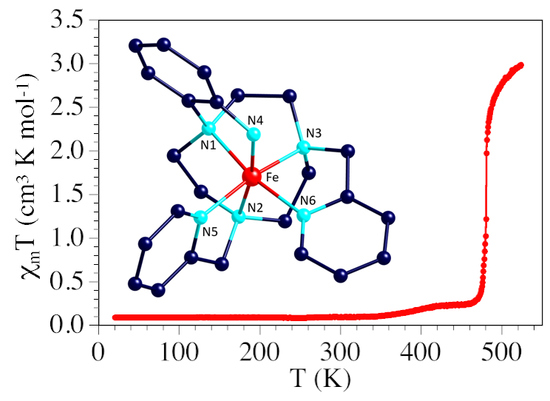
2.3. Magnetic Properties
2.4. Thermogravimetric (TG) and Differential Scanning Calorimetry (DSC) Measurements
2.5. Comparison with Other Related Complexes
3. Experimental Section
3.1. Starting Materials
3.2. Synthesis of 1-(2-Nitrophenyl)-4,7-bis(pyridin-2-ylmethyl)-1,4,7-triazacyclononane (B)
3.3. Synthesis of 1-(2-Aminophenyl)-4,7-bis(pyridin-2-ylmethyl)-1,4,7-triazacyclononane (L6)
3.4. Synthesis of [Fe(L6)](ClO4)2 (1)
3.5. Characterization of the Materials
3.6. Magnetic Measurements
3.7. Thermogravimetric (TG) and Differential Scanning Calorimetry (DSC) Measurements
3.8. Crystallographic Data Collection and Refinement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, K.S.; Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Shalabaeva, V.; Ridier, K.; Rat, S.; Manrique-Juarez, M.D.; Salmon, L.; Séguy, I.; Rotaru, A.; Molnár, G.; Bousseksou, A. Room temperature current modulation in large area electronic junctions of spin crossover thin films. Appl. Phys. Lett. 2018, 112, 013301. [Google Scholar] [CrossRef]
- Dugay, J.; Giménez-Marqués, M.; Kozlova, T.; Zandbergen, H.W.; Coronado, E.; van der Zant, H.S.J. Spin Switching in Electronic Devices Based on 2D Assemblies of Spin-Crossover Nanoparticles. Adv. Mater. 2015, 27, 1288–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, I.-R.; Park, J.G.; Haney, C.R.; Harris, T.D. Spin crossover iron(II) complexes as PARACEST MRI thermometers. Chem. Sci. 2014, 5, 2461–2465. [Google Scholar] [CrossRef]
- Baadji, N.; Sanvito, S. Giant Resistance Change across the Phase Transition in Spin-Crossover Molecules. Phys. Rev. Lett. 2012, 108, 217201. [Google Scholar] [CrossRef] [PubMed]
- Prins, F.; Monrabal-Capilla, M.; Osorio, E.A.; Coronado, E.; van der Zant, H.S.J. Room-Temperature Electrical Addressing of a Bistable Spin-Crossover Molecular System. Adv. Mater. 2011, 23, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Bousseksou, A.; Negre, N.; Goiran, M.; Salmon, L.; Tuchagues, J.P.; Boillot, M.L.; Boukheddaden, K.; Varret, F. Dynamic triggering of a spin-transition by a pulsed magnetic field. Eur. Phys. J. B 2000, 13, 451–456. [Google Scholar]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Goodwin, H.A. (Eds.) Spin Crossover in Transition Metal Compounds; Springer: Berlin/Heidelberg, Germany, 2004; Volume 233–235. [Google Scholar]
- Linares, J.; Codjovi, E.; Garcia, Y. Pressure and Temperature Spin Crossover Sensors with Optical Detection. Sensors 2012, 12, 4479–4492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gütlich, P.; Gaspar, A.B.; Garcia, Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jureschi, C.-M.; Linares, J.; Boulmaali, A.; Dahoo, P.R.; Rotaru, A.; Garcia, Y. Pressure and Temperature Sensors Using Two Spin Crossover Materials. Sensors 2016, 16, 187. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. (Ed.) Spin-Crossover Materials, Properties and Applications; John Wiley & Sons Ltd.: Oxford, UK, 2013. [Google Scholar]
- Mekuimemba, A.D.; Conan, F.; Mota, A.-J.; Palacios, M.-A.; Colacio, E.; Triki, S. On the Magnetic Coupling and Spin Crossover Behavior in Complexes Containing the Head-to-Tail [FeII2(μ-SCN)2] Bridging Unit: A Magnetostructural Experimental and Theoretical Study. Inorg. Chem. 2018, 57, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Pittala, N.; Thétiot, F.; Triki, S.; Boukheddaden, K.; Chastanet, G.; Marchivie, M. Cooperative 1D Triazole-Based Spin Crossover FeII Material With Exceptional Mechanical Resilience. Chem. Mater. 2017, 29, 490–494. [Google Scholar] [CrossRef]
- Phan, H.; Hrudka, J.J.; Igimbayeva, D.; Lawson Daku, L.M.; Shatruk, M. A Simple Approach for Predicting the Spin State of Homoleptic Fe(II) Tris-diimine Complexes. J. Am. Chem. Soc. 2017, 139, 6437–6447. [Google Scholar] [CrossRef] [PubMed]
- Pittala, N.; Thétiot, F.; Charles, C.; Triki, S.; Boukheddaden, K.; Chastanet, G.; Marchivie, M. An unprecedented trinuclear FeII triazole-based complex exhibiting a concerted and complete sharp spin transition above room temperature. Chem. Commun. 2017, 53, 8356–8359. [Google Scholar] [CrossRef] [PubMed]
- Milin, E.; Patinec, V.; Triki, S.; Bendeif, E.-E.; Pillet, S.; Marchivie, M.; Chastanet, G.; Boukheddaden, K. Elastic Frustration Triggering Photoinduced Hidden Hysteresis and Multistability in a Two-Dimensional Photoswitchable Hofmann-Like Spin-Crossover Metal–Organic Framework. Inorg. Chem. 2016, 55, 11652–11661. [Google Scholar] [CrossRef] [PubMed]
- Shatruk, M.; Phan, H.; Chrisostomo, B.A.; Suleimenova, A. Symmetry-breaking structural phase transitions in spin crossover complexes. Coord. Chem. Rev. 2015, 289–290, 62–73. [Google Scholar] [CrossRef]
- Atmani, A.; El Hajj, F.; Benmansour, S.; Marchivie, M.; Triki, S.; Conan, F.; Patinec, V.; Handel, H.; Dupouy, G.; Gómez-García, C.J. Guidelines to design new spin crossover materials. Coord. Chem. Rev. 2010, 254, 1559–1569. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Monrabal-Capilla, M.; García-Martínez, J.; Pardo-Ibáñez, P. Bistable Spin-Crossover Nanoparticles Showing Magnetic Thermal Hysteresis near Room Temperature. Adv. Mater. 2007, 19, 1359–1361. [Google Scholar] [CrossRef]
- Bianchi, A.; Micheloni, M.; Paoletti, P. Thermodynamic aspects of the polyazacycloalkane complexes with cations and anions. Coord. Chem. Rev. 1991, 110, 17–113. [Google Scholar] [CrossRef]
- Lord, R.L.; Schultz, F.A.; Baik, M.-H. Spin Crossover-Coupled Electron Transfer of [M(tacn)2]3+/2+ Complexes (tacn = 1,4,7-Triazacyclononane; M = Cr, Mn, Fe, Co, Ni). J. Am. Chem. Soc. 2009, 131, 6189–6197. [Google Scholar] [CrossRef] [PubMed]
- Krüger, H.-J. Spin transition in octahedral metal complexes containing tetraazamacrocyclic ligands. Coord. Chem. Rev. 2009, 253, 2450–2459. [Google Scholar] [CrossRef]
- Long, N.J.; Parker, D.G.; Speyer, P.R.; White, A.J.P.; Williams, D.J. Synthesis, characterisation and polymerisation of vinylbenzene-substituted triazacyclododecanes and their transition metal complexes. J. Chem. Soc. Dalton Trans. 2002, 10, 2142–2150. [Google Scholar] [CrossRef]
- Blakesly, A.W.; Payne, S.C.; Hagen, K.S. Spin-State Variation in Solid State and Solution of Mononuclear Iron(II) 1,4,7-Trimethyl-1,4,7-triazacyclonane Complexes. Inorg. Chem. 2000, 39, 1979–1989. [Google Scholar] [CrossRef]
- Walf, A.H.; Benda, R.; Litterst, F.J.; Stebani, U.; Schmidt, S.; Lattermann, G. Liquid Crystalline Octahedral Iron(III) Complexes with 1,4,7-Tris[3,4-bis(decyloxy)benzyl]-1,4,7-triazacyclononane: Thermal Characterization and Mössbauer Investigations of Bridging and Redox Behavior. Chem. Eur. J. 1998, 4, 93–99. [Google Scholar] [CrossRef]
- Fallis, I.A.; Griffiths, P.C.; Hibbs, D.E.; Hursthouse, M.B.; Winnington, A.L. Solid state and solution behaviour of novel transition metal containing surfactants. Chem. Commun. 1998, 6, 665–666. [Google Scholar] [CrossRef]
- Farrugia, L.J.; Lovatt, P.A.; Peacock, R.D. Synthesis of a series of novel binucleating ligands based on 1,4,7-triazacyclononane and o-, m- and p-xylene: Crystal structure of the µ-hydroxy-bridged dicopper(II) complex [Cu2Lm(OH)2][BPh4]2 [Lm = α,α′-bis (N-1,4,7-triazacyclononane)-m-xylene]. J. Chem. Soc. Dalton Trans. 1997, 6, 911–912. [Google Scholar] [CrossRef]
- Turner, J.W.; Schultz, F.A. Solution Characterization of the Iron(II) Bis(1,4,7-Triazacyclononane) Spin-Equilibrium Reaction. Inorg. Chem. 2001, 40, 5296–5298. [Google Scholar] [CrossRef] [PubMed]
- Dabrowiak, J.C.; Merrel, P.H.; Busch, D.H. High- and low-spin six-coordinate complexes of iron(II) with a saturated tetradentate macrocyclin ligand. Inorg. Chem. 1972, 11, 1979–1988. [Google Scholar] [CrossRef]
- Goedken, V.L.; Merrell, P.H.; Busch, D.H. Complexes of Iron (II) and Iron (III) with the Tetradentate Macrocycle 5,7,7,12,14,14-Hexamethyl-1,4,8,11-tetraazacyclotetradeca-4,11-diene. J. Am. Chem. Soc. 1972, 94, 3397–3405. [Google Scholar] [CrossRef]
- Lindoy, L.F. The Chemistry of Macrocyclic Ligand Complexes; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Parker, D. (Ed.) Macrocycle Synthesis: A Practical Approach; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Fensterbank, A.; Zhu, J.; Riou, D.; Larpent, C. A convenient one-step synthesis of mono-N-functionalized tetraazamacrocycles. J. Chem. Soc. Perkin Trans. 1999, 1, 811–816. [Google Scholar] [CrossRef]
- Helps, A.M.; Parker, D.; Morphy, J.R.; Chapman, J. General routes for the synthesis of mono, di and tri-N-substituted derivatives of cyclam. Tetrahedron 1989, 45, 219–226. [Google Scholar] [CrossRef]
- Royal, G.; Dahaoui-Gindrey, V.; Dahaoui, S.; Tabard, A.; Guilard, R.; Pullumbi, P.; Lecomte, C. New Synthesis of trans-Disubstituted Cyclam Macrocycles—Elucidation of the Disubstitution Mechanism on the Basis of X-ray Data and Molecular Modeling. Eur. J. Org. Chem. 1998, 1998, 1971–1975. [Google Scholar] [CrossRef]
- Davies, P.J.; Taylor, M.R. Formation of 1,11-bis(pendant donor)-cyclam derivatives via the formamidinium salt (cyclam = 1,4,8,11-tetraazacyclotetradecane). Chem. Commun. 1998, 7, 827–828. [Google Scholar] [CrossRef]
- Bellouard, F.; Chuburu, F.; Kervarec, N.; Toupet, L.; Triki, S.; Le Mest, Y.; Handel, H. cis-Diprotected cyclams and cyclens: A new route to symmetrically or asymmetrically 1,4-disubstituted tetraazamacrocycles and to asymmetrically tetrasubstituted derivatives. J. Chem. Soc. Perkin Trans. 1999, 1, 3499–3505. [Google Scholar] [CrossRef]
- Mishra, A.K.; Draillard, K.; Faivre-Chauvet, A.; Gestin, J.-F.; Curtet, C.; Chatal, J.-F. A convenient, novel approach for the synthesis of polyaza macrocyclic bifunctional chelating agents. Tetrahedron Lett. 1996, 37, 7515–7518. [Google Scholar] [CrossRef]
- El Hajj, F.; Sebki, G.; Patinec, V.; Marchivie, M.; Triki, S.; Handel, H.; Yefsah, S.; Tripier, R.; Gómez-García, C.J.; Coronado, E. Macrocycle-Based Spin-Crossover Materials. Inorg. Chem. 2009, 48, 10416–10423. [Google Scholar] [CrossRef] [PubMed]
- Milin, E.; Benaicha, B.; El Hajj, F.; Patinec, V.; Triki, S.; Marchivie, M.; Gómez-García, C.J.; Pillet, S. Magnetic Bistability in Macrocycle-Based FeII Spin-Crossover Complexes: Counter Ion and Solvent Effects. Eur. J. Inorg. Chem. 2016, 34, 5305–5314. [Google Scholar] [CrossRef]
- Drahoš, B.; Trávničk, Z. Spin crossover Fe(II) complexes of a cross-bridged cyclam derivative. Dalton Trans. 2018, 47, 6134–6145. [Google Scholar] [CrossRef] [PubMed]
- Vithanarachchi, S.M.; Kovacs, D.; Borbas, K.E. Synthesis and photophysical characterization of luminescent lanthanide complexes of nucleotide-functionalized cyclen- and dipicolinate-based ligands. Inorg. Chim. Acta 2017, 460, 148–158. [Google Scholar] [CrossRef] [Green Version]
- El Hajj, F.; Patinec, V.; Triki, S.; Handel, H.; Marchivie, M. Isomerism as a remarkable tool for the design of new potentially bridging macrocycle ligands: Synthesis and characterization of the [Cu(4-L1)](NO3)2·2H2O polymeric chain (4-L1=mono-N (4-picolyl)cyclen). Inorg. Chem. Commun. 2010, 13, 1314–1316. [Google Scholar] [CrossRef]
- Batsanov, A.S.; Goeta, A.E.; Howard, J.A.K.; Maffeo, D.; Puschmann, H.; Williams, J.A.G. Nickel(II) complexes of the isomeric tetraazamacrocyclic ligands 1,11- and 1,8-bis(2-pyridylmethyl)-cyclam and of a structurally constrained N4,N8-methylene bridged analogue. Polyhedron 2001, 20, 981–986. [Google Scholar] [CrossRef]
- Comba, P.; Luther, S.M.; Maas, O.; Pritzkow, H.; Vielfort, A. Template Synthesis of a Tetraazamacrocyclic Ligand with Two Pendant Pyridinyl Groups: Properties of the Isomers of the Metal-Free Ligand and of Their First-Row Transition Metal Compounds. Inorg. Chem. 2001, 40, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.-H.; Lu, S.-L.; Zhang, R.-H.; Liu, H.; Zhu, H.-P.; Liu, Q.-T. Synthesis, characterization and crystal structures of the cobalt(II) and iron(II) complexes with an octadentate ligand, 1,4,7,10-tetrakis(2-pyridylmethyl)-1,4,7,10-tetraazacyclododecane (L), [ML]2+. Polyhedron 2000, 19, 431–435. [Google Scholar]
- Bu, X.-H.; Chen, W.; Mu, L.-J.; Zhang, Z.-H.; Zhang, R.-H.; Clifford, T. Syntheses, crystal structures and properties of new manganese(II) complexes with macrocyclic polyamine ligands bearing pyridyl donor pendants. Polyhedron 2000, 19, 2095–2100. [Google Scholar] [CrossRef]
- Christiansen, L.; Hendrickson, D.N.; Toftlund, H.; Wilson, S.R.; Xie, C.L. Synthesis and structure of metal complexes of triaza macrocycles with three pendant pyridylmethyl arms. Inorg. Chem. 1986, 25, 2813–2818. [Google Scholar] [CrossRef]
- Shepherd, A.J.; Rosa, P.; Fallis, I.A.; Guionneau, P.; Howard, J.A.K.; Goeta, A.E. Structural origin of the gradual spin transition in a mononuclear iron(II) complex. J. Phys. Chem. Solids 2012, 73, 193–197. [Google Scholar] [CrossRef]
- Al-Obaidi, A.H.R.; McGarvey, J.J.; Taylor, K.P.; Bell, S.E.J.; Jensen, K.B.; Toftlund, H. Observation of biphasic kinetics in light-induced spin-state crossover in an iron(II) complex in solution. J. Chem. Soc. Chem. Commun. 1993, 6, 536–538. [Google Scholar] [CrossRef]
- Touti, F.; Maurin, P.; Canaple, L.; Beuf, O.; Hasserodt, J. Awakening of a Ferrous Complex’s Electronic Spin in an Aqueous Solution Induced by a Chemical Stimulus. Inorg. Chem. 2012, 51, 31–33. [Google Scholar] [CrossRef] [PubMed]
- McGowan, P.C.; Temple, C.N. New N-monofunctionalised 1,4,7-triazacyclononane complexes of iron. Inorg. Chim. Acta 2009, 362, 3165–3171. [Google Scholar] [CrossRef]
- Stavila, V.; Allali, M.; Canaple, L.; Stortz, Y.; Franc, C.; Maurin, P.; Beuf, O.; Dufay, O.; Samarut, J.; Janier, M.; et al. Significant relaxivity gap between a low-spin and a high-spin iron(II) complex of structural similarity: An attractive off–on system for the potential design of responsive MRI probes. New J. Chem. 2008, 32, 428–435. [Google Scholar] [CrossRef]
- Gott, A.L.; McGowan, P.C.; Podesta, T.J.; Thornton-Pett, M. Pendant arm N-monofunctionalised 1,4,7-triazacyclononane complexes of Fe(II) and Ru(II). J. Chem. Soc. Dalton Trans. 2002, 18, 3619–3623. [Google Scholar] [CrossRef]
- Fallis, I.A.; Farley, R.D.; Abdul Malik, K.M.; Murphy, D.M.; Smith, H.J. Divalent first-row transition metal complexes of the rigid pendant-arm ligand 1,4,7-tris(2-aminophenyl)-1,4,7-triazacyclononane. J. Chem. Soc. Dalton Trans. 2000, 20, 3632–3639. [Google Scholar] [CrossRef]
- Spiccia, L.; Fallon, G.D.; Grannas, M.J.; Nichols, P.J.; Tiekink, E.R.T. Synthesis and characterization of mononuclear and binuclear iron(II) complexes of pentadentate and bis(pentadentate) ligands derived from 1,4,7-triazacyclononane. Inorg. Chim. Acta 1998, 279, 192–199. [Google Scholar] [CrossRef]
- de Martino Norante, G.; Di Vaira, M.; Mani, F.; Mazzi, S.; Stoppioni, P. Transition metal complexes of a functionalised triazamacrocycle. J. Chem. Soc. Dalton Trans. 1992, 3, 361–365. [Google Scholar] [CrossRef]
- Guillou, A.; Lima, L.M.P.; Roger, M.; Esteban-Gómez, D.; Delgado, R.; Platas-Iglesias, C.; Patinec, V.; Tripier, R. 1,4,7-Triazacyclononane-Based Bifunctional Picolinate Ligands for Efficient Copper Complexation. Eur. J. Inorg. Chem. 2017, 2017, 2435–2443. [Google Scholar] [CrossRef]
- Roger, A.; Lima, L.M.P.; Frindel, M.; Platas-Iglesias, C.; Gestin, J.F.; Delgado, R.; Patinec, V.; Tripier, R. Monopicolinate-dipicolyl Derivative of Triazacyclononane for Stable Complexation of Cu2+ and 64Cu2+. Inorg. Chem. 2013, 52, 5246–5259. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Tjioe, L.; Graham, B.; Belousoff, M.J.; Juran, S.; Walther, M.; Künstler, J.U.; Bergmann, R.; Stephan, H.; Spiccia, L. Synthesis, Copper(II) Complexation, 64Cu-Labeling, and Bioconjugation of a New Bis(2-pyridylmethyl) Derivative of 1,4,7-Triazacyclononane. Bioconjugate Chem. 2008, 19, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Arends, I.W.C.E.; Gamez, P.; Sheldon, R.A. Green oxidation of alcohols using biomimetic Cu complexes and Cu enzymes as catalysts. Adv. Inorg. Chem. 2006, 58, 235–279. [Google Scholar]
- Belle, C.; Rammal, W.; Pierre, J.L. Sulfur ligation in copper enzymes and models. Inorg. Biochem. 2005, 99, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Boulatov, R. Understanding the reaction that powers this world: Biomimetic studies of respiratory O2 reduction by cytochrome oxidase. Pure Appl. Chem. 2004, 76, 303–319. [Google Scholar] [CrossRef]
- Himes, R.A.; Karlin, K.D. Copper–dioxygen complex mediated C–H bond oxygenation: Relevance for particulate methane monooxygenase (pMMO). Curr. Opin. Chem. Biol. 2009, 13, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Tseberlidis, G.; Intrieri, D.; Caselli, A. Catalytic Applications of Pyridine-Containing Macrocyclic Complexes. Eur. J. Inorg. Chem. 2017, 30, 3589–3603. [Google Scholar] [CrossRef]
- Guionneau, P.; Marchivie, M.; Bravic, G.; Létard, J.-F.; Chasseau, D. Structural Aspects of Spin Crossover. Example of the [FeIILn(NCS)2] Complexes. Top. Curr. Chem. 2004, 234, 97–128. [Google Scholar]
- Roger, A.; Patinec, V.; Tripier, R.; Triki, S.; Le Poul, N.; Le Mest, Y. Synthesis of an unsymmetrical N-functionalized triazacyclononane ligand and its Cu(II) complex. Inorg. Chim. Acta 2014, 417, 201–207. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Oxford Diffraction. Xcalibur CCD/RED CrysAlis Software System; Oxford-Diffraction Ltd.: Abingdon, UK, 2006. [Google Scholar]
- Sheldrick, A. SHELX97. In Program for Crystal Structure Analysis; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]





| 1 | |
|---|---|
| Formula | C24H30Cl2FeN6O8 |
| F. Wt. | 657.29 |
| Space group | P21/c |
| Crystal system | Monoclinic |
| a/Å | 9.3491(11) |
| b/Å | 13.8911(17) |
| c/Å | 20.308(2) |
| α/ ° | 90 |
| β/° | 91.734(11) |
| γ/° | 90 |
| V/Å3 | 2636.2(5) |
| Z | 4 |
| T/(K) | 150 |
| ρcalc/g cm−3 | 1.656 |
| μ/mm−1 | 0.837 |
| F(000) | 1360 |
| θ range (deg) | 3.346–25.236 |
| Total reflections | 10476 |
| Unique reflections | 4754 |
| R(int) | 0.0899 |
| Data with I > 2σ(I) | 2712 |
| Nvar | 407 |
| R1 a on I > 2σ(I) | 0.0698 |
| wR2b (all) | 0.1613 |
| GooF c on F2 | 1.015 |
| Δρmax (eÅ−3) | 0.488 |
| Δρmin (eÅ−3) | −0.495 |
| Atoms | Distance | Atoms | Angle | Atoms | Angle | Atoms | Angle |
|---|---|---|---|---|---|---|---|
| Fe-N1 | 2.002(5) | N1-Fe-N2 | 86.4(2) | N2-Fe-N3 | 85.6(2) | N3-Fe-N5 | 168.2(2) |
| Fe-N2 | 1.998(5) | N1-Fe-N3 | 86.0(2) | N2-Fe-N4 | 170.00(19) | N3-Fe-N6 | 84.1(2) |
| Fe-N3 | 1.999(4) | N1-Fe-N4 | 84.2(2) | N2-Fe-N5 | 82.90(17) | N4-Fe-N5 | 94.66(17) |
| Fe-N4 | 2.005(5) | N1-Fe-N5 | 96.14(17) | N2-Fe-N6 | 98.58(19) | N4-Fe-N6 | 91.28(19) |
| Fe-N5 | 1.999(4) | N1-Fe-N6 | 168.49(17) | N3-Fe-N4 | 97.2(2) | N5-Fe-N6 | 94.76(17) |
| Fe-N6 | 1.973(5) |
| Fe(II) Complex | [FeL6](ClO4)2 (1) | [FeL5](ClO4)2 (2) | [FeL8](ClO4)2 (3) |
|---|---|---|---|
| T (K) | 150 | 298 | 120 |
| Fe-N(tacn) | 2.002(5) | 2.001(6) | 2.001(2) |
| 1.998(5) | 2.001(6) | 1.999(2) | |
| 1.999(4) | 2.001(6) | 1.996(2) | |
| Fe-N(py) | 1.999(4) | 1.979(6) | - |
| 1.973(5) | 1.979(6) | - | |
| - | 1.979(6) | - | |
| Fe-N(aniline) | 2.005(5) | - | 2.053(2) |
| - | - | 2.037(2) | |
| - | - | 2.035(2) | |
| <d(Fe-N)> | 1.996(5) | 1.990(6) | 2.020(2) |
| aΣ (°) | 63(1) | 64(1) | 56(1) |
| Magnetic behavior | SCO | LS (T < 406 K) * | Gradual SCO |
| T1/2 > 425 K | T1/2 = 281 K | ||
| Reference | This work | 50 | 51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halit, M.; Roger, M.; Patinec, V.; Yefsah, S.; Gómez-García, C.J.; Triki, S. Spin Cross-Over (SCO) Complex Based on Unsymmetrical Functionalized Triazacyclononane Ligand: Structural Characterization and Magnetic Properties. Magnetochemistry 2019, 5, 19. https://doi.org/10.3390/magnetochemistry5010019
Halit M, Roger M, Patinec V, Yefsah S, Gómez-García CJ, Triki S. Spin Cross-Over (SCO) Complex Based on Unsymmetrical Functionalized Triazacyclononane Ligand: Structural Characterization and Magnetic Properties. Magnetochemistry. 2019; 5(1):19. https://doi.org/10.3390/magnetochemistry5010019
Chicago/Turabian StyleHalit, Merzouk, Mélissa Roger, Véronique Patinec, Said Yefsah, Carlos J. Gómez-García, and Smail Triki. 2019. "Spin Cross-Over (SCO) Complex Based on Unsymmetrical Functionalized Triazacyclononane Ligand: Structural Characterization and Magnetic Properties" Magnetochemistry 5, no. 1: 19. https://doi.org/10.3390/magnetochemistry5010019
APA StyleHalit, M., Roger, M., Patinec, V., Yefsah, S., Gómez-García, C. J., & Triki, S. (2019). Spin Cross-Over (SCO) Complex Based on Unsymmetrical Functionalized Triazacyclononane Ligand: Structural Characterization and Magnetic Properties. Magnetochemistry, 5(1), 19. https://doi.org/10.3390/magnetochemistry5010019