Magnetic Nanoparticles: An Overview for Biomedical Applications
Abstract
:1. Introduction
2. Composition and Properties of Magnetic Nanoparticles
3. Synthesis Methods
4. Characterization
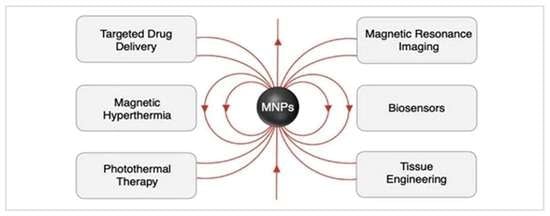
5. Biomedical Applications
5.1. Magnetically-Guided Drug Delivery
5.1.1. In Vitro Studies
5.1.2. In Vivo Studies
5.2. Magnetic Hyperthermia
5.3. Photothermal Therapy (PTT)
5.4. Magnetic Resonance Imaging (MRI)
5.5. Magnetic Particle Imaging (MPI)
5.6. Biosensors
5.7. Tissue Engineering
6. Conclusions and Future Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Gandhi, S.; Roy, I. Drug delivery applications of casein nanostructures: A minireview. J. Drug Deliv. Sci. Technol. 2021, 66, 102843. [Google Scholar] [CrossRef]
- Zhang, H.W.; Liu, Y.; Sun, S.H. Synthesis and assembly of magnetic nanoparticles for information and energy storage appli-cations. Front. Phys. China 2010, 5, 347–356. [Google Scholar] [CrossRef]
- Zahn, M. Magnetic fluid and nanoparticle applications to nanotechnology. J. Nanopart. Res. 2001, 3, 73–78. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Chen, M.; Gu, H.; Rapole, S.B.; Pallavkar, S.; Ho, T.C.; Hopper, J.; Guo, Z. Magnetic nanocomposites for envi-ronmental remediation. Adv. Powder Technol. 2013, 24, 459–467. [Google Scholar] [CrossRef]
- Krug, B.; Asumadu, J. Magnetic nanoparticle-based gyroscopic detection device: A review. In Proceedings of the IEEE Interna-tional Conference on Industrial Technology, Taipei, Taiwan, 14–17 March 2016. [Google Scholar]
- Zhang, Q.; Yang, X.; Guan, J. Applications of magnetic nanomaterials in heterogeneous catalysis. ACS Appl. Nano Mater. 2019, 2, 4681–4697. [Google Scholar] [CrossRef]
- Vaseem, M.; Ghaffar, F.A.; Farroqui, M.F.; Shamim, A. Iron Oxide Nanoparticle-Based Magnetic Ink Development for Fully Printed Tunable Radio-Frequency Devices. Adv. Mater. Technol. 2018, 3, 1700242. [Google Scholar] [CrossRef]
- Chen, M.L.; He, Y.J.; Chen, X.W.; Wang, J.H. Quantum dots conjugated with Fe3O4-filled carbon nanotubes for cancer-targeted imaging and magnetically guided drug delivery. Langmuir 2012, 28, 16469–16477. [Google Scholar] [CrossRef]
- Bi, Q.; Song, X.; Hu, A.; Luo, T.; Jin, R.; Ai, H.; Nie, Y. Magnetofection: Magic magnetic nanoparticles for efficient gene deliv-ery. Chin. Chem. Lett. 2020, 31, 3041–4046. [Google Scholar] [CrossRef]
- Leong, S.S.; Ahmad, Z.; Low, S.C.; Camacho, J.; Faraudo, J.; Lim, J. Unified View of Magnetic Nanoparticle Separation under Magnetophoresis. Langmuir 2020, 36, 8033–8055. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.E.; Medina, R.H.; Fernandez, V.V.A.; Garcia-Casillas, P.E. Magnetic heating ability of silica-cobalt ferrite nanopar-ticles. Rev. Mex. Ing. Quim. 2014, 13, 555–561. [Google Scholar]
- Kim, E.H.; Lee, H.S.; Kwak, B.K.; Kim, B.K. Synthesis of ferrofluid with magnetic nanoparticles by sonochemical method for MRI contrast agent. J. Magn. Magn. Mater. 2005, 289, 328–330. [Google Scholar] [CrossRef]
- Gil, S.; Mano, J.F. Magnetic composite biomaterials for tissue engineering. Biomater. Sci. 2014, 2, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Laneros-Mendez, S. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Zhu, X.; Li, L.; Tang, J.; Yang, C.; Yu, H.; Liu, K.; Zheng, Z.; Gu, X.; Yu, Q.; Xu, F.J.; et al. Cascade-responsive nano-assembly for efficient photothermal-chemo synergistic inhibition of tumor metastasis by targeting cancer stem cells. Biomaterials 2022, 280, 121305. [Google Scholar] [CrossRef]
- Chang, T.; Qiu, Q.; Ji, A.; Qu, C.; Chen, H.; Cheng, Z. Organic single molecule based nano-platform for NIR-II imaging and chemo-photothermal synergistic treatment of tumor. Biomaterials 2022, 287, 121670. [Google Scholar] [CrossRef]
- Zuo, X.; Xu, H.; Zhang, J.; Sui, Y.; Fang, T.; Zhang, D. Carbothermal treated ferrite nanoparticles with improved magnetic heating efficiency and T1-MRI performance. J. Magn. Magn. Mater. 2022, 548, 168999. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef]
- Martins, P.M.; Lima, A.C.; Ribeiro, S.; Lanceros-Mendez, S.; Martins, P. Magnetic Nanoparticles for Biomedical Applications: From the Soul of the Earth to the Deep History of Ourselves. ACS Appl. Bio Mater. 2021, 4, 5839–5870. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, E. Magnetic nanoparticles in targeted drug delivery: A review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735. [Google Scholar] [CrossRef]
- Gandhi, S.; Roy, I. Synthesis and characterization of manganese ferrite nanoparticles, and its interaction with bovine serum albumin: A spectroscopic and molecular docking approach. J. Mol. Liq. 2019, 296, 111871. [Google Scholar] [CrossRef]
- Dehsari, H.S.; Ksenofontov, V.; Möller, A.; Jakob, G.; Asadi, K. Determing magnetite/maghemite composition and core-shell nanostructure from magnetization curve for iron oxide nanoparticles. J. Phys. Chem. C 2018, 122, 28292–28301. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Dias, H.V.R.; Kharissova, O.V.; Jiménez-Pérez, V.M.; Pérez, B.O.; Flores, B.M. Iron-containing nanomaterials: Synthesis, properties, and environmental applications. RSC Adv. 2012, 2, 9325–9358. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003; pp. 15–38. [Google Scholar]
- Gaikwad, R.S.; Chae, S.Y.; Mane, R.S.; Han, S.H.; Joo, O.S. Cobalt ferrite nanocrystallites for sustainable hydrogen production application. Int. J. Electrochem. 2011, 6, 729141. [Google Scholar] [CrossRef]
- Lu, H.C.; Chang, J.E.; Vong, W.W.; Chen, H.T.; Chen, Y.L. Porous ferrite synthesis and catalytic effect on benzene degradation. Int. J. Phys. Sci. 2011, 6, 855–865. [Google Scholar]
- Braga, T.P.; Sales, B.M.C.; Pinheiro, A.N.; Herrera, W.T.; Saitovitch, E.B.; Valentini, A. Catalytic properties of cobalt and nickel ferrites dispersed in mesoporous silicon oxide for ethylbenzenedehydrogenation with CO2. Catal. Sci. Technol. 2011, 1, 1383–1392. [Google Scholar] [CrossRef]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef]
- Chouly, C.; Pouliquen, D.; Lucet, I.; Jeune, J.J.; Jallet, P. Development of superparamagnetic nanoparticles for MRI: Effect of particle size, charge, and surface nature on biodistribution. J. Microencapsul. 1996, 13, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Nishikawa, M.; Ohtsubo, Y.; Ohno, J.; Takakura, Y.; Sezaki, H.; Hashida, M. Control of in vivo ate of albumin derivatives utilizing combined chemical modification. J. Drug Target. 1994, 2, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kush, P.; Kumar, P.; Singh, R.; Kaushik, A. Aspects of high-performance and bio-acceptable magnetic nanoparticles for biomedical application. Asian J. Pharm. Sci. 2021, 16, 704–737. [Google Scholar] [CrossRef] [PubMed]
- Markides, H.; Rotherham, M.; El Haj, A.J. Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. J. Nanomater. 2012, 2012, 614094. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Weissleder, R.; Stark, D.D.; Engelstad, B.L.; Bacon, B.R.; Compton, C.C.; White, D.L.; Jacobs, P.; Lewis, J. Superparamagnetic iron oxide: Pharmacokinetics and toxicity. AJR Am. J. Roentgenol. 1989, 152, 167–173. [Google Scholar] [CrossRef]
- Gokduman, K.; Bestepe, F.; Li, L.; Yarmush, M.L.; Usta, O.B. Dose-, treatment- and time-dependent toxicity of superparamagnetic iron oxide nanoparticles on primary rat hepatocytes. Nanomedicine 2018, 13, 11. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Rezaee, M. Magnetic nanoparticles: Synthesis, stabilization, functionalization, characterization, and applications. J. Iran. Chem. Soc. 2010, 7, 1–37. [Google Scholar] [CrossRef]
- Gandhi, S.; Issar, S.; Mahapatro, A.K.; Roy, I. Cobalt ferrite nanoparticles for bimodal hyperthermia and their mechanistic interactions with lysozyme. J. Mol. Liq. 2020, 310, 113194. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Soundararajan, D.; Kim, K.H. Synthesis of CoFe2O4 magnetic nanoparticles by thermal decomposition. J. Magn. 2014, 19, 5–9. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, K.; Wu, W.; Cui, X.; Li, Y. Magnetic properties of nanocrystalline CuFe2O4 and kinetics of thermal decomposition of precursor. J. Therm. Anal. Calorim. 2011, 111, 9–16. [Google Scholar] [CrossRef]
- Choi, C.J.; Dong, X.L.; Kim, B.K. Microstructure and magnetic properties of Fe nanoparticles synthesized by chemical vapor condensation. Mater. Trans. 2001, 42, 2046–2049. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.; Shen, M. Hydrothermal synthesis and functionalization of iron oxide nanoparticles for MR imaging applications. Part. Part. Syst. Charact. 2014, 31, 1223–1237. [Google Scholar] [CrossRef]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, F.; Hassanzadeh-Tabrizi, S.A.; Bigham, A. In situ microemulsion synthesis of hydroxyapatite-MgFe2O4 nanocomposites as a magnetic drug delivery system. Mater. Sci. Eng. C 2016, 68, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W. Microwave-induced polyol-process synthesis of MIIFe2O4 (M = Mn, Co) nanoparticles and magnetic property. Mater. Chem. Phys. 2008, 108, 227–231. [Google Scholar] [CrossRef]
- Ang, K.H.; Alexandrou, I.; Mathur, N.D.; Amaratunga, G.A.J.; Haq, S. The effect of carbon encapsulation on the magnetic properties of Ni nanoparticles produced by arc discharge in de-ionized water. Nanotechnology 2004, 15, 520–524. [Google Scholar] [CrossRef]
- Khan, A.A.; Khan, S.; Khan, S.; Rentschler, S.; Laufer, S.; Deigner, H.P. Biosynthesis of iron oxide magnetic nanoparticles using clinically isolated Pseudomonas aeruginosa. Sci. Rep. 2021, 11, 20503. [Google Scholar] [CrossRef]
- Cabrera, L.; Gutierrez, S.; Menendez, N.; Morales, M.P.; Herrasti, P. Magnetite nanoparticles: Electrochemical synthesis and characterization. Electrochim. Acta 2008, 53, 3436–3441. [Google Scholar] [CrossRef]
- Weissleder, R.; Bogdanov, A.; Neuwelt, E.A.; Papisov, M. Long-circulating iron oxides for MR imaging. Adv. Drug Dev. Rev. 1995, 16, 321–334. [Google Scholar] [CrossRef]
- Kievit, F.M.; Veiseh, O.; Bhattarai, N.; Fang, C.; Gunn, J.W.; Lee, D.; Ellenbogen, R.G.; Olson, J.M.; Zhang, M. PEI-PEG-Chitosan copolymer coated iron oxide nanoparticles for safe gene delivery: Synthesis, complexation, and transfection. Adv. Funct. Mater. 2009, 19, 2244–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Wang, J.; Niu, G.; Huang, J.; Chen, K.; Li, X.; Chen, X. Human serum albumin coated iron oxide nanoparticles for efficient celllabeling. Chem. Commun. 2010, 46, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef]
- Lu, Y.; Yin, Y.; Mayers, B.T.; Xia, Y. Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol-gel approach. Nano Lett. 2002, 2, 183–186. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Chen, H.; Tang, J.; Nie, L. Sonochemical synthesis, structure and magnetic properties of air-stable Fe3O4/Au nanoparticles. Nanotechnology 2007, 18, 145609. [Google Scholar] [CrossRef]
- Park, J.B.; Jeong, S.H.; Jeong, M.S.; Kim, J.Y.; Cho, B.K. Synthesis of carbon-encapsulated magnetic nanoparticles by pulsed laser irradiation of solution. Carbon 2008, 46, 1369–1377. [Google Scholar] [CrossRef]
- Martina, M.S.; Fortin, J.P.; Ménager, C.; Clément, O.; Barratt, G.; Grabielle-Madelmont, C.; Gazeau, F.; Cabuil, V.; Lesieur, S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J. Am. Chem. Soc. 2005, 127, 10676–10685. [Google Scholar] [CrossRef]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef]
- Liu, J.; Kitamoto, Y. Influence of silica coating process on fine structure and magnetic properties of iron oxide nanoparticles. Electrochim. Acta 2015, 183, 148–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Ozkan, M.; Ozkan, C.S. Magnetic force microscopy of iron oxide nanoparticles and their cellular uptake. Biotechnol. Prog. 2009, 25, 923–928. [Google Scholar] [CrossRef] [PubMed]
- De Jaeger, N.; Demeyere, H.; Finsy, R.; Sneyers, R.; Vanderdeelan, J.; van der Meeren, P.; van Laethem, M. Particle sizing by photon correlation spectroscopy part I: Monodisperse lattices: Influence of scattering angle and concentration of dispersed material. Part. Part. Syst. Charact. 1991, 8, 179–186. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on recent progress in magnetic nanoparticles: Synthesis, characterization, and diverse applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Joos, A.; Rümenapp, C.; Wagner, F.E.; Gleich, B. Characterization of iron oxide nanoparticles by Mössbauer spectroscopy at ambient temperature. J. Magn. Magn. Mater. 2016, 339, 123–129. [Google Scholar] [CrossRef]
- Grass, R.N.; Athanassiou, E.K.; Stark, W.J. Covalently functionalized cobalt nanoparticles as a platform for magnetic separations in organic synthesis. Angew. Chem. Int. Ed. 2007, 46, 4909–4912. [Google Scholar] [CrossRef]
- Shen, L.; Laibinis, P.E.; Hatton, T.A. Bilayer surfactant stabilized magnetic fluids: Synthesis and interactions at interfaces. Langmuir 1999, 15, 447–453. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, Y.; Wang, T.; Cai, Y.; Jiang, G. Preparation of silica-magnetite nanoparticle mixed hemimicelle sorbents for extraction of several typical phenolic compounds from environmental water samples. J. Chromatogr. A 2008, 1188, 140–147. [Google Scholar] [CrossRef]
- Hu, H.; Yuan, Y.; Lim, S.; Wang, C.H. Phase structure dependence of magnetic behaviour in iron oxide nanorods. Mater. Des. 2020, 185, 108241. [Google Scholar] [CrossRef]
- Zahid, M.; Nadeem, N.; Hanif, M.A.; Bhatti, I.A.; Bhatti, H.N.; Mustafa, G. Metal ferrites and their graphene-based nanocomposites: Synthesis, characterization, and applications in wastewater treatment. In Magnetic Nanostructures; Abd-Elsalam, K.A., Mohamed, M.A., Prasad, R., Eds.; Springer: Berlin/Heidelgerg, Germany, 2019; pp. 181–212. [Google Scholar]
- Díaz-Pardo, R.; Valenzuela, R. Characterization of Magnetic Phases in Nanostructured Ferrites by Electron Spin Resonance. In Advanced Electromagnetic Waves; Bashir, S.O., Ed.; IntechOpen: London, UK, 2015. [Google Scholar]
- Singh, A.K.; Srivastava, O.N.; Singh, K. Shape and size-dependent magnetic properties of Fe3O4 nanoparticles synthesized using piperidine. Nanoscale Res. Lett. 2017, 12, 298. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Panmand, R.; Kumar, G.; Mahajan, S.M.; Kale, B.B. Magneto-optic evaluation of antiferromagnetic α-Fe2O3 nanoparticles coated on a quartz substrate. Int. Soc. Opt. Photonics 2016, 12, 97580O. [Google Scholar]
- Pang, C.L.K.; Lee, K. Hyperthermia in Oncology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 18–53. [Google Scholar]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Arias, J.L.; Gallardo, V.; Ruiz, M.A.; Delgado, A.V. Magnetite/poly(alkylcyanoacrylate) (core/shell) nanoparticles as 5-Fluorouracil delivery systems for active targeting. Eur. J. Pharm. Biopharm. 2008, 69, 54–63. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. Engl. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Shubayev, V.I.; Pisanic II, T.R.; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Del. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Jori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Kettering, M.; Winter, J.; Zeisberger, M.; Bremer-Streck, S.; Oehring, H.; Bergemann, C.; Alexiou, C.; Hergt, R.; Halbhuber, K.J.; Kaiser, W.A.; et al. Magnetic nanoparticles as bimodal tools in magnetically induced labelling and magnetic heating of tumour cells: An in vitro study. Nanotechnology 2007, 18, 175101. [Google Scholar] [CrossRef]
- Chorny, M.; Hood, E.; Levy, R.J.; Muzykantov, V.R. Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles. J. Control. Release 2010, 146, 144–151. [Google Scholar] [CrossRef]
- Chiang, W.H.; Ho, V.T.; Chen, H.H.; Huang, W.C.; Huang, Y.F.; Lin, S.C.; Chern, C.S.; Chiu, H.C. Superparamagnetic Hollow Hybrid Nanogels as a Potential Guidable Vehicle System of Stimuli-Mediated MR Imaging and Multiple Cancer Therapeutics. Langmuir 2013, 29, 6434–6443. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Linemann, T.; Pondman, K.M.; Lichota, J.; Kim, K.S.; Pieters, R.J.; Visser, G.M.; Moos, T. Uptake and Transport of Superparamagnetic Iron Oxide Nanoparticles through Human Brain Capillary Endothelial Cells. ACS Chem. Neurosci. 2013, 4, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.W.; Kanwal, F.; Batool, A.; Jamil, T.; Zia-ul-Haq, M.; Ijaz, B.; Huang, Q.; Ullah, Z. Polymer-coated CoFe2O4 nanoassemblies as biocompatible magnetic nanocarriers for anticancer drug delivery. J. Mater. Sci. 2017, 52, 9282–9293. [Google Scholar] [CrossRef]
- Child, H.W.; del Pino, P.A.; de la Fuente, J.M.; Hursthouse, A.S.; Stirling, D.; Mullen, M.; McPhee, G.M.; Nixon, C.; Jayawarna, V.; Berry, C.C. Working Together: The Combined Application of a Magnetic Field and Penetratin for the Delivery of Magnetic Nanoparticles to Cells in 3D. ACS Nano 2011, 5, 7910–7919. [Google Scholar] [CrossRef] [PubMed]
- Widder, K.J.; Senyei, A.E.; Scarpelli, D.G. Magnetic Microspheres: A Model System for Site Specific Drug Delivery in Vivo. Proc. Soc. Exp. Biol. Med. 1978, 158, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Lübbe, A.S.; Bergemann, C.; Huhnt, W.; Fricke, T.; Riess, H.; Brock, J.W.; Huhn, D. Preclinical experiences with magnetic drug targeting: Tolerance and efficacy. Cancer Res. 1996, 56, 4694–4701. [Google Scholar]
- Jurgons, R.; Seliger, C.; Hilpert, A.; Trahms, L.; Odenbach, S.; Alexiou, C. Drug loaded magnetic nanoparticles for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2893–S2902. [Google Scholar] [CrossRef]
- Tietze, R.; Lyer, S.; Dürr, S.; Struffert, T.; Engelhorn, T.; Schwarz, M.; Eckert, E.; Göen, T.; Vasylyev, S.; Peukert, W.; et al. Efficient drug-delivery using magnetic nanoparticles—Biodistribution and therapeutic effects in tumour bearing rabbits. Nanomedicine 2013, 9, 961–971. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Zhang, Z.R.; Yang, H.; Tan, Q.Y.; Qin, S.R.; Qiu, X.L. Lyophilized Paclitaxel Magnetoliposomes as a Potential Drug Delivery System for Breast Carcinoma via Parenteral Administration: In Vitro and in Vivo Studies. Pharm. Res. 2005, 22, 573–583. [Google Scholar] [CrossRef]
- Béalle, G.; Corato, R.D.; Kolosnjaj-Tabi, J.; Dupuis, V.; Clément, O.; Gazeau, F.; Wilhelm, C.; Ménager, C. Ultra Magnetic Liposomes for MR Imaging, Targeting, and Hyperthermia. Langmuir 2012, 28, 11834–11842. [Google Scholar] [CrossRef]
- Chertok, B.; David, A.E.; Yang, V.C. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administration. Biomaterials 2010, 31, 6317–6324. [Google Scholar] [CrossRef]
- Aryan, H.; Beigzadeh, B.; Siavashi, M. Euler-Lagrange numerical simulation of improved magnetic drug delivery in a three-dimensional CT-based carotid artery bifurcation. Comput. Methods Programs Biomed. 2022, 219, 106778. [Google Scholar] [CrossRef]
- Mah, C.; Fraites, T.J.; Zolotukhin, I.; Song, S.; Flotte, T.R.; Dobson, J.; Batich, C.; Byrne, B.J. Improved Method of Recombinant AAV2 Delivery for Systemic Targeted Gene Therapy. Mol. Ther. 2002, 6, 106–112. [Google Scholar] [CrossRef]
- Shen, J.M.; Guan, X.M.; Liu, X.Y.; Lan, J.F.; Cheng, T.; Zhang, H.X. Luminescent/magnetic hybrid nanoparticles with folate-conjugated peptide composites for tumor-targeted drug delivery. Bioconj. Chem. 2012, 23, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Yellen, B.B.; Forbes, Z.G.; Halverson, D.S.; Fridman, G.; Barbee, K.A.; Chorny, M.; Levy, R.; Friedman, G. Targeted drug delivery to magnetic implants for therapeutic applications. J. Magn. Magn. Mater. 2005, 293, 647–654. [Google Scholar]
- Pouponneau, P.; Leroux, J.P.; Soulez, G.; Gaboury, L.; Martel, S. Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation. Biomaterials 2011, 32, 3481–3486. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010, 20, 8760–8767. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [PubMed]
- Psimadas, D.; Baldi, G.; Ravagli, C.; Comes, F.M.; Locatelli, C.; Innocenti, C.; Sangregorio, C.; Loudos, G. Comparison of the magnetic, radiolabeling, hyperthermic and biodistribution properties of hybrid nanoparticles bearing CoFe2O4 and Fe3O4 metal cores. Nanotechnology 2014, 25, 25101. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Nkambule, T.T.I.; Mamba, B.B. Spinel ferrite nanoparticles and nanocomposites for biomedical applications and their toxicity. Mater. Sci. Eng. C 2020, 107, 110314. [Google Scholar]
- Huong, L.; Nam, N.H.; Doan, D.H.; My Nhung, H.T.; Quang, B.T.; Nam, P.H.; Thong, P.Q.; Phuc, N.X.; Thu, H.P. Folate attached, curcumin loaded Fe3O4 nanoparticles: A novel multifunctional drug delivery system for cancer treatment. Mater. Chem. Phys. 2016, 172, 98–104. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Huang, L.Y.; Yang, M.C.; Liu, T.Y.; Tsai, S.C.; Yang, C.Y.; Kuo, C.Y.; Chan, T.Y.; Zou, H.M.; Lian, W.N.; et al. Magnetic liposomes for colorectal cancer cells therapy by high-frequency magnetic field treatment. Nanoscale Res. Lett. 2014, 9, 497. [Google Scholar] [CrossRef]
- Gandhi, S.; Roy, I. Methylene blue loaded, silica coated cobalt ferrite nanoparticles with potential for combination therapy. Mater. Res. Express 2019, 6, 7. [Google Scholar] [CrossRef]
- Kahil, H.; El Sayed, H.M.; Elsayed, E.M.; Sallam, A.M.; Talaat, M.; Sattar, A.A. Effect of in vitro magnetic fluid hyperthermia using citrate coated cobalt ferrite nanoparticles on tumor cell death. Rom. J. Biophys. 2015, 25, 209–224. [Google Scholar]
- Oh, Y.; Moorthy, M.S.; Manivasagan, P.; Bharathiraja, S.; Oh, J. Magnetic hyperthermia and pH-responsive effective drug delivery to the sub-cellular level of human breast cancer cells by modified CoFe2O4 nanoparticles. Biochimie 2017, 133, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.B.; Silvestri, N.; Fernandez-Cabada, T.; Marinaro, F.; Fernandes, S.; Fiorito, S.; Miscuglio, M.; Serantes, D.; Ruta, S.; Livesey, K.; et al. Exploiting Unique Alignment of Cobalt Ferrite Nanoparticles, Mild Hyperthermia, and Controlled Intrinsic Cobalt Toxicity for Cancer Therapy. Adv. Mater. 2020, 32, 2003712. [Google Scholar] [CrossRef]
- Iatridi, Z.; Vamvakidis, K.; Tsougos, I.; Vassiou, K.; Dendrinou-Samara, C.; Bokias, G. Multifunctional Polymeric Platform of Magnetic Ferrite Colloidal Superparticles for Luminescence, Imaging, and Hyperthermia Applications. ACS Appl. Mater. Interfaces 2016, 8, 35059–35070. [Google Scholar] [CrossRef]
- Yang, J.C.; Chen, Y.; Li, Y.H.; Yin, X.B. Magnetic Resonance Imaging-Guided Multi-Drug Chemotherapy and Photothermal Synergistic Therapy with pH and NIR-Stimulation Release. ACS Appl. Mater. Interfaces 2017, 9, 22278–22288. [Google Scholar] [CrossRef]
- Iqbal, Y.; Bae, H.; Rhee, I.; Hong, S. Magnetic heating of silica-coated manganese ferrite nanoparticles. J. Magn. Magn. Mater. 2016, 409, 80–86. [Google Scholar] [CrossRef]
- Ghutepatil, P.R.; Salunkhe, A.B.; Khot, V.M.; Pawar, S.H. APTES (3-aminopropyltriethoxy silane) functionalized MnFe2O4 nanoparticles: A potential material for magnetic fluid hyperthermia. Chem. Pap. 2019, 73, 2189–2197. [Google Scholar] [CrossRef]
- Shen, S.; Kong, F.; Guo, X.; Wu, L.; Shen, H.; Xie, M.; Wang, X.; Jin, Y.; Ge, Y. CMCTS stabilized Fe3O4 particles with extremely low toxicity as highly efficient near-infrared photothermal agents for in vivo tumor ablation. Nanoscale 2013, 5, 8056–8066. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Shao, Y.; Peng, J.; Dai, X.; Li, H.; Wu, Q.; Shi, D. Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials 2013, 34, 4078–4088. [Google Scholar] [CrossRef]
- Chen, H.; Burnett, J.; Zhang, F.; Zhang, J.; Paholak, H.; Sun, D. Highly crystallized iron oxide nanoparticles as effective and biodegradable mediators for photothermal cancer therapy. J. Mater. Chem. B 2014, 2, 757–765. [Google Scholar] [CrossRef]
- Espinosa, A.; Corato, R.D.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [PubMed]
- Terreno, E.; Castelli, D.D.; Viale, A.; Aime, S. Challenges for Molecular Magnetic Resonance Imaging. Chem. Rev. 2010, 110, 3019–3042. [Google Scholar] [CrossRef] [PubMed]
- Rümenapp, C.; Gleich, B.; Haase, A. Magnetic Nanoparticles in Magnetic Resonance Imaging and Diagnostics. Pharm. Res. 2012, 29, 1165–1179. [Google Scholar] [PubMed]
- Chee, H.L.; Gan, C.R.R.; Ng, M.; Low, L.; Fernig, D.G.; Bhakoo, K.K.; Paramelle, D. Biocompatible Peptide-Coated Ultrasmall Superparamagnetic Iron Oxide Nanoparticles for In Vivo Contrast-Enhanced Magnetic Resonance Imaging. ACS Nano 2018, 12, 6480–6491. [Google Scholar]
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; van de Kaa, C.H.; de la Rosette, J.; Weissleder, R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Engl. J. Med. 2003, 348, 2491–2499. [Google Scholar]
- Varallyay, P.; Nesbit, G.; Muldoon, L.L.; Nixon, R.R.; Delashaw, J.; Cohen, J.I.; Petrillo, A.; Rink, D.; Neuwelt, E.A. Comparison of two superparamagnetic viral-sized iron oxide particles ferumoxides and ferumoxtran-10 with a gadolinium chelate in imaging intracranial tumors. AJNR Am. J. Neuroradiol. 2002, 23, 510–519. [Google Scholar]
- Nahrendorf, M.; Jaffer, F.A.; Kelly, K.A.; Sosnovik, D.E.; Aikawa, E.; Libby, P.; Weissleder, R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 2006, 114, 1504–1511. [Google Scholar]
- Bulte, J.W.M.; Kraitchman, D.L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004, 17, 484–499. [Google Scholar]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022. [Google Scholar]
- Hayashi, K.; Sato, Y.; Sakamoto, W.; Yogo, T. Theranostic Nanoparticles for MRI-Guided Thermochemotherapy: “Tight” Clustering of Magnetic Nanoparticles Boosts Relaxivity and Heat-Generation Power. ACS Biomater. Sci. Eng. 2017, 3, 95–105. [Google Scholar]
- Gao, Z.; He, T.; Zhang, P.; Li, X.; Zhang, Y.; Lin, J.; Hao, J.; Huang, P.; Cui, J. Polypeptide-Based Theranostics with Tumor-Microenvironment-Activatable Cascade Reaction for Chemo-ferroptosis Combination Therapy. ACS Appl. Mater. Interfaces 2020, 12, 20271–20280. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.S.; Hsieh, M.R.; Liu, R.S.; Wei, D.H.; Hsiao, M. Magnetically Guided Theranostics: Optimizing Magnetic Resonance Imaging with Sandwich-Like Kaolinite-Based Iron/Platinum Nanoparticles for Magnetic Fluid Hyperthermia and Chemotherapy. Chem. Mater. 2020, 32, 697–708. [Google Scholar] [CrossRef]
- Gleich, B.; Weizenecker, J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.Y.; Bishop, M.; Zheng, B.; Ferguson, R.M.; Khandhar, A.P.; Kemp, S.J.; Krishnan, K.M.; Goodwill, P.W.; Conolly, S.M. Magnetic Particle Imaging: A Novel in Vivo Imaging Platform for Cancer Detection. Nano Lett. 2017, 17, 1648–1654. [Google Scholar] [PubMed] [Green Version]
- Bauer, L.M.; Situ, S.F.; Griswold, M.A.; Samia, A.C.S. Magnetic Particle Imaging Tracers: State-of-the-Art and Future Directions. J. Phys. Chem. Lett. 2015, 6, 2509–2517. [Google Scholar] [CrossRef]
- Song, G.; Chen, M.; Zhang, Y.; Cui, L.; Qu, H.; Zheng, X.; Wintermark, M.; Liu, Z.; Rao, J. Janus Iron Oxides @ Semiconducting Polymer Nanoparticle Tracer for Cell Tracking by Magnetic Particle Imaging. Nano Lett. 2018, 18, 182–189. [Google Scholar]
- Szwargulski, P.; Wilmes, M.; Javidi, E.; Thieben, F.; Graeser, M.; Koch, M.; Gruettner, C.; Adam, G.; Gerloff, C.; Magnus, T.; et al. Monitoring Intracranial Cerebral Hemorrhage Using Multicontrast Real-Time Magnetic Particle Imaging. ACS Nano 2020, 14, 13913–13923. [Google Scholar] [CrossRef]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar]
- Wang, C.; Wang, C.; Wang, X.; Wang, K.; Zhu, Y.; Rong, Z.; Wang, W.; Xiao, R.; Wang, S. Magnetic SERS Strip for Sensitive and Simultaneous Detection of Respiratory Viruses. ACS Appl. Mater. Interfaces 2019, 11, 19495–19505. [Google Scholar]
- Masud, M.K.; Yadav, S.; Islam, M.N.; Nguyen, N.T.; Salomon, C.; Kline, R.; Alamri, H.R.; Alothman, Z.A.; Yamauchi, Y.; Hossain, M.S.A.; et al. Gold-Loaded Nanoporous Ferric Oxide Nanocubes with Peroxidase-Mimicking Activity for Electrocatalytic and Colorimetric Detection of Autoantibody. Anal. Chem. 2017, 89, 11005–11013. [Google Scholar] [CrossRef]
- Kim, M.S.; Kweon, S.H.; Cho, S.; An, S.S.A.; Kim, M.I.; Doh, J.; Lee, J. Pt-Decorated Magnetic Nanozymes for Facile and Sensitive Point-of-Care Bioassay. ACS Appl. Mater. Interfaces 2017, 9, 35133–35140. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Kamihira, M. Tissue Engineering Using Magnetite Nanoparticles. In Progress in Molecular Biology and Translational Science, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 355–395. [Google Scholar]
- Cartmell, S.H.; Dobson, J.; Verschueren, S.B.; El Haj, A.J. Development of magnetic particle techniques for long-term culture of bone cells with intermittent mechanical activation. IEEE Trans. Nanobiosci. 2002, 1, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Shibata, R.; Numaguchi, Y.; Kito, T.; Suzuki, H.; Shimizu, K.; Ito, A.; Honda, H.; Murohara, T. Enhanced angiogenesis by transplantation of mesenchymal stem cell sheet created by a novel magnetic tissue engineering method. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2210–2215. [Google Scholar] [PubMed]
- Kito, T.; Shibata, R.; Ishii, M.; Suzuki, H.; Himeno, T.; Kataoka, Y.; Yamamura, Y.; Yamamoto, T.; Nishio, N.; Ito, S.; et al. iPS cell sheets created by a novel magnetite tissue engineering method for reparative angiogenesis. Sci. Rep. 2013, 3, 1418. [Google Scholar]
- Yang, H.Y.; Jang, M.S.; Gao, G.H.; Lee, J.H.; Lee, D.S. pH-Responsive biodegradable polymeric micelles with anchors to interface magnetic nanoparticles for MR imaging in detection of cerebral ischemic area. Nanoscale 2016, 8, 12588–12598. [Google Scholar] [PubMed]
- Carvalho, S.M.; Leonel, A.G.; Mansur, A.A.P.; Carvalho, I.C.; Krambrock, K.; Mansur, H.S. Bifunctional magnetopolymersomes of iron oxide nanoparticles and carboxymethylcellulose conjugated with doxorubicin for hyperthermo-chemotherapy of brain cancer cells. Biomater. Sci. 2019, 7, 2102–2122. [Google Scholar]
- Price, D.N.; Stromberg, L.R.; Kunda, N.K.; Muttil, P. In Vivo Pulmonary Delivery and Magnetic-Targeting of Dry Powder Nano-in-Microparticles. Mol. Pharm. 2017, 14, 4741–4750. [Google Scholar] [CrossRef]
- Cho, M.H.; Lee, E.J.; Son, M.; Lee, J.H.; Yoo, D.; Kim, J.W.; Park, S.W.; Shin, J.S.; Cheon, J. A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nat. Mater. 2012, 11, 1038–1043. [Google Scholar]
- Feng, Q.; Zhang, Y.; Zhang, W.; Hao, Y.; Wang, Y.; Zhang, H.; Hou, L.; Zhang, Z. Programmed near-infrared light-responsive drug delivery system for combined magnetic tumor-targeting magnetic resonance imaging and chemo-phototherapy. Acta Biomater. 2017, 49, 402–413. [Google Scholar]
- Wang, Y.; Zou, L.; Qiang, Z.; Jiang, J.; Zhu, Z.; Ren, J. Enhancing Targeted Cancer Treatment by Combining Hyperthermia and Radiotherapy Using Mn–Zn Ferrite Magnetic Nanoparticles. ACS Biomater. Sci. Eng. 2020, 6, 3550–3562. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zheng, D.; Lin, X.; Wei, Z.; Zhang, D.; Li, Z.; Zhang, Y.; Wu, M.; Liu, X. Cancer cell membrane-coated magnetic nanoparticles for MR/NIR fluorescence dual-modal imaging and photodynamic therapy. Biomater. Sci. 2018, 6, 1834–1845. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Su, C.M.; Yeh, J.C.; Wu, P.R.; Tsai, T.Y.; Lou, S.L. Synthesis of composite magnetic nanoparticles Fe3O4 with alendronate for osteoporosis treatment. Int. J. Nanomed. 2016, 11, 4583–4594. [Google Scholar] [CrossRef] [PubMed]
- Geilich, B.M.; Gelfat, I.; Sridhar, S.; van de Ven, A.L.; Webster, T.J. Superparamagnetic iron oxide-encapsulating polymersome nanocarriers for biofilm eradication. Biomaterials 2017, 119, 78–85. [Google Scholar] [CrossRef]
- Wang, C.; Gu, B.; Liu, Q.; Pang, Y.; Xiao, R.; Wang, S. Combined use of vancomycin-modified Ag-coated magnetic nanoparticles and secondary enhanced nanoparticles for rapid surface-enhanced Raman scattering detection of bacteria. Int. J. Nanomed. 2018, 13, 1159–1178. [Google Scholar] [CrossRef]
- Lee, C.N.; Wang, Y.M.; Lai, W.F.; Chen, T.J.; Yu, M.C.; Fang, C.L.; Yu, F.L.; Tsai, Y.H.; Chang, W.H.S.; Zuo, C.S.; et al. Super-paramagnetic iron oxide nanoparticles for use in extrapulmonary tuberculosis diagnosis. Clin. Microbiol. Infect. 2012, 18, E149–E157. [Google Scholar]
- He, Y.; Wang, Y.; Yang, X.; Xie, S.; Yuan, R.; Chai, Y. Metal Organic Frameworks Combining CoFe2O4 Magnetic Nanoparticles as Highly Efficient SERS Sensing Platform for Ultrasensitive Detection of N-Terminal Pro-Brain Natriuretic Peptide. ACS Appl. Mater. Interfaces 2016, 8, 7683–7690. [Google Scholar] [CrossRef]
- Materia, M.E.; Leal, M.P.; Scotto, M.; Balakrishnan, P.B.; Avugadda, S.K.; García-Martín, M.L.; Cohen, B.E.; Chan, E.M.; Pellegrino, T. Multifunctional Magnetic and Upconverting Nanobeads as Dual Modal Imaging Tools. Bioconj. Chem. 2017, 28, 2707–2714. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Sharmin, S.; Nasrin, F.; Yamazaki, M.; Abe, F.; Suzuki, T.; Park, E.Y. Use of Target-Specific Liposome and Magnetic Nanoparticle Conjugation for the Amplified Detection of Norovirus. ACS Appl. Bio Mater. 2020, 3, 3560–3568. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, S.; Ren, F.; Chen, L.; Zeng, J.; Zhu, M.; Cheng, Z.; Gao, M.; Li, Z. Ultrasmall Magnetic CuFeSe2 Ternary Nanocrystals for Multimodal Imaging Guided Photothermal Therapy of Cancer. ACS Nano 2017, 11, 5633–5645. [Google Scholar]
- Cha, B.G.; Jeong, H.G.; Kang, D.W.; Nam, M.J.; Kim, C.K.; Kim, D.Y.; Choi, I.Y.; Ki, S.K.; Kim, S.I.; Han, J.H.; et al. Customized lipid-coated magnetic mesoporous silica nanoparticle doped with ceria nanoparticles for theragnosis of intracerebral hemorrhage. Nano Res. 2018, 11, 3582–3592. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, H.; Song, W.; Ru, X.; Zhou, W.; Yu, X. A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Chen, G.; Liu, B.; Deng, X.; Wei, Y.; Xia, J.; Xing, B.; Ma, P.; Lin, J. Synthesis and Optimization of MoS2@Fe3O4-ICG/Pt(IV) Nanoflowers for MR/IR/PA Bioimaging and Combined PTT/PDT/Chemotherapy Triggered by 808 nm Laser. Adv. Sci. 2017, 4, 1600540. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Lina, X.; Changxuan, W.; Cong, L.; Xiaolan, Y.; Tao, H.; Hong, A. Orthogonal test design for the optimization of superparamagnetic chitosan plasmid gelatin microspheres that promote vascularization of artificial bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1439–1449. [Google Scholar] [CrossRef]
- Najafipour, A.; Gharieh, A.; Fassihi, A.; Sadeghi-Aliabadi, H.; Mahdavian, A.R. MTX-Loaded Dual Thermoresponsive and pH-Responsive Magnetic Hydrogel Nanocomposite Particles for Combined Controlled Drug Delivery and Hyperthermia Therapy of Cancer. Mol. Pharm. 2021, 18, 275–284. [Google Scholar] [CrossRef] [PubMed]










| Synthesis Method | Procedure | Merits | Demerits | Ref. |
|---|---|---|---|---|
| Co-precipitation | magnetic particles precipitated out from stoichiometric mixture of metal ions aqueous solution by addition of base | hydrophilic NPs; simple and scalable | broader size distribution | [40] |
| Thermal decomposition | organometallic precursor decomposed in non-polar boiling solvent at high temperatures in presence of stabilizing agent | monodisperse and size-controlled NPs; high yield | hydrophobic non-biocompatible NPs; toxic solvents | [42] |
| Hydrothermal | magnetic nanoparticles synthesized from aqueous metal solutions at high temperatures (>100 °C) and high pressure (>1 atm) in presence of an oxidizing agent | crystalline and water dispersible NPs of high purity | slow; large reaction time | [46] |
| Chemical vapor condensation | heating volatile metal precursors in an inert atmosphere | easy to prepare; small size NPs | precursors are toxic; specialized instruments required | [45] |
| Reverse microemulsion | particles formed inside hydrophilic core of micelles formed from surfactant molecules | simple room temperature synthesis; size controllability | low yield | [48] |
| Microwave | microwave-assisted heating of metal salt solutions in presence of oxidizing agent/base | homogenous heating and fast process | small scale production; expensive equipment | [49] |
| Electrochemical | magnetic particles synthesized from dissolution of sacrificial metal anode | size controllability by varying current density and potential | small scale production; broad size distribution | [52] |
| Characterization Technique | Application |
|---|---|
| Transmission electron microscopy (TEM) | morphology, size distribution, thickness of coating |
| Scanning electron microscopy (SEM) | surface morphology, size distribution |
| Atomic force microscopy (AFM) | size, morphology, surface roughness |
| Dynamic light scattering (DLS) | hydrodynamic size, polydispersity |
| Energy dispersive X-ray spectroscopy (EDS) | elemental composition, elemental mapping |
| X-ray fluorescence spectroscopy (XFS) | qualitative and quantitative elemental analysis |
| Atomic absorption spectroscopy (AAS) | qualitative and quantitative elemental analysis |
| Inductively coupled plasma mass spectroscopy (ICP-MS) | elemental composition |
| Mössbauer spectroscopy | differentiating between magnetite and maghemite |
| X-ray diffraction (XRD) spectroscopy | crystalline structure |
| Selected area electron diffraction (SAED) | crystal structure, lattice parameters |
| Zeta potential | surface charge |
| Fourier-transform infrared (FT-IR) spectroscopy | surface functionality, presence of coating |
| Thermal gravimetric analysis (TGA) | thermal stability, presence of coating |
| Vibrating sample magnetometer (VSM) | change in magnetization as function of external magnetic field |
| Superconducting quantum interference device (SQUID) | change in magnetization as function of temperature and external magnetic field |
| Electron paramagnetic resonance (EPR) spectroscopy | characterization of magnetic phases |
| Physical-property measurement system (PPMS) | direct or alternating current (DC/AC) magnetization measurement as a function of temperature and/or applied magnetic field |
| Kerr spectroscopy | imaging differences in magnetization on the magnetic material’s surface |
| Magnetic Nanoparticles Formulation | Composition | Size (nm) | Biomedical Application | In Vitro/In Vivo Application | Ref. |
|---|---|---|---|---|---|
| Fe3O4-loaded mPEG-b-P(DPA-DE)LG micelles | hydrophobic SPIONs encapsulated in hydrophobic core of mPEG-b-P(DPA-DE)LG micelles; Dopamine (DPA) acted as anchor for SPIONs for facilitating self-assembly of polymer | DLS: 147 ± 3 TEM: ~120 | pH-responsive nanocarrier for MRI | Rat model with cerebral ischemia | [142] |
| MION@CMC-DOX | MIONs stabilized and coated by biocompatible CMC layer and bioconjugated with DOX via amide bonds | DLS: 38 ± 2 TEM: 7 ± 2 | combined chemotherapy and magnetic hyperthermia | HEK 293T and U87 cells | [143] |
| Nano-in-microparticles (NIMs) | dry powder containing SPIONs and DOX in lactose matrix | DLS: NA TEM: 1000–5000 | magnetically-targeted pulmonary delivery | A549 cells; Male Balb/c mice | [144] |
| Zinc doped iron oxide NPs conjugated to DR4 Ab | thiolated Zn0.4Fe2.6O4 NPs conjugated with protein A through sulpho-SMCC crosslinker; protein A binds with the Fc region of DR4 Ab | DLS: 32 TEM: NA | non-invasive approach to turn “on” apoptosis cell signalling using magnetic field | DLD-1 cells; zebrafish | [145] |
| HMCuS/DOX@IONP-PEG | DOX loaded positively charged hollow mesoporous CuS NPs capped with negatively charged IONPs and further surface conjugated with PEG | DLS: 124.5 ± 3.8 TEM: NA | multimodal system for combined chemotherapy, MRI, and NIR-light activated PTT and PDT | MCF-7 cells; tumor bearing mice | [146] |
| Hyaluronic acid-modified Mn-Zn ferrite (HA-MZF) nanoparticles | Mn0.6Zn0.4Fe2O4 (MZF) NP encapsulated in micelles using NH2-PEG2000-PCL3400 to form MZF-NH2 micelles and further surface modified with HA using EDC/NHS crosslinking reaction | DLS: 195 TEM: NA | receptor (CD44) mediated targeted delivery with combined hyperthermia and radiotherapy | A549 cells; Male Balb/c mice | [147] |
| Photosensitizer (Ce6) and cancer cell membrane (CCM) coated SSAP (SSAP-Ce6@CCM) | oleic acid coated SPIONs embedded in crosslinked matrix of styrene and AA, and further modified with PEI using carboxyl groups of AA; Ce6 adsorbed onto the surface and finally cloaked with CCM | DLS: 192 TEM: NA | PDT and dual-modal MR/NIR fluorescence imaging | SMMC-7721 tumor-bearing mice | [148] |
| Bisphosphonate conjugated dextran-coated Fe3O4 (Bis/Dex/Fe3O4) NPs | bisphosphonate (alendronate) covalently attached by EDC/NHS crosslinking to carboxyl surface modified dextran-coated Fe3O4 NPs | DLS: NA TEM: 20 | radiofrequency-induced thermolysis of osteoclasts for treatment of osteoporosis | male Wistar rats | [149] |
| Methicillin and SPIONs containing nano polymerosome | hydrophobic SPIONs and hydrophilic methicillin containing multi-compartment nano polymerosome made up of amphiphilic dieblock co-polymer (mPEG-PDLLA) | DLS: 83 ± 6 TEM: NA | penetration of Staphylococcus epidermidis biofilm with the aid of magnetic field | male Wistar rats | [150] |
| Fe3O4@Ag-Van MNPs | cationic PEI modified Fe3O4 NPs were coated with Ag colloidal NPs to form Fe3O4@Ag NPs; vancomycin (Van) was conjugated to carboxyl modified Fe3O4@Ag NPs | DLS: NA TEM: 290 | SERS-based biosensor for detecting and capturing bacteria | S. aureus 04018, E. coli BL21 strains | [151] |
| Mtb (Mycobacterium tuberculosis) antibody (MTBsAb) conjugated SPIONs | dextran-coated on SPIONs crosslinked by epichlorohydrin; treated with EDBE to generate amino groups at dextran ends followed by addition of SA to form SPIO-EDBE-SA; finally conjugated with MtbsAb | DLS: NA TEM: 3.8 ± 0.4 | ultrasensitive MR imaging for diagnosis of extrapulmonary tuberculosis | C57BL/6 mice bearing Mtb granulomas | [152] |
| Antibody (Ab1) conjugated Au functionalized CoFe2O4 (CoFe2O4@AuNPs@Ab1) NPs | primary antibodies (Ab1) immobilized by Au NPs functionalized CoFe2O4 NPs | DLS: NA TEM: 80 | SERS-based immunosensor for detecting NT-proBNP (biomarker for heart failure) | - | [153] |
| Magnetic (IONP) and upconverting (UCNP) nanobeads (MUCNBs) | IONPs and UCNPs simultaneously incorporated by wrapping in amphiphilic poly(maleic anhydride-alt-1-octadecene) polymer | DLS: 120 ± 10 TEM: NA | NIR fluorescence imaging and T2 contrast agents | HeLa-WT and A431 cells | [154] |
| Calcein−liposome/virus (NoV)/Fe3O4 nanoconjugates | APTES functionalized Fe3O4 NPs and calcein liposome conjugated separately with NoV-specific antibody form a sandwich-like structure in presence of NoV | DLS: 1120 TEM: NA | fluorometric detection of Norovirus (NoV) | - | [155] |
| CuFeSe2 −99mTc nanocrystals | CuFeSe2 nanocrystals fabricated in presence of trithiol-terminated poly-(methacrylic acid) and further labeled with radioactive 99mTc | DLS: NA TEM: 4.1 ± 0.4 | Multimodal imaging (photoacoustic, magnetic resonance and computed tomography imaging) PTT | 4T1 cells; 4T1 tumor-bearing BALB/c mice | [156] |
| Lipid-coated Fe3O4 mesoporous silica NPs doped with ceria NPs | ceria NPs loaded on surface of mesoporous silica NPs embedded with Fe3O4 NPs and further encapsulated by a lipid bilayer | DLS: ~250 TEM: ~100 | MRI and anti-inflammatory effect by scavenging ROS for theragnosis of intracerebral hemorrhage | RAW 264.7 cells; male Sprague-Dawley rats | [157] |
| Poly (amino ester) coated Fe3O4 NPs | poly (amino ester) having carboxyl groups coated on Fe3O4 NPs | DLS: NA TEM: 10.22 ± 2.8 | viral-RNA extraction method for detection of SARS-CoV-2 | - | [158] |
| MoS2@Fe3O4-ICG/Pt(IV) nanoflowers | PEI functionalized MoS2 nanoflowers covalently grafted with Fe3O4 NPs and further loaded with ICG and Pt(IV) prodrugs | DLS: NA TEM: 80 | multimodal infrared thermal/photoacoustic/magnetic resonance imaging with combined PDT, PTT, and chemotherapy | L929 cells; H22 tumor-bearing Balb/c mice | [159] |
| Superparamagnetic (Fe3O4) chitosan plasmid gelatin microspheres | superparamagnetic chitosan-coated Fe3O4 NPs attached with (pReceiver-M29-VEGF165/DH5a) plasmids embedded in gelatin microspheres | DLS: NA TEM: NA | neovascularization in artificial bone scaffolds on application of magnetic field | white rabbits | [160] |
| Core–shell Fe3O4 poly(NIPAM-co-DEAEMA) magnetic hydrogel composites loaded with methotrexate | Fe3O4 NPs (core) surface modified with TMSPMA encapsulated in poly(NIPAM-co-DEAEMA) shell and loaded with methotrexate | DLS: NA TEM: 50–70 | dual pH- and thermo- responsive drug delivery and hyperthermia | MCF-7 cells | [161] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittal, A.; Roy, I.; Gandhi, S. Magnetic Nanoparticles: An Overview for Biomedical Applications. Magnetochemistry 2022, 8, 107. https://doi.org/10.3390/magnetochemistry8090107
Mittal A, Roy I, Gandhi S. Magnetic Nanoparticles: An Overview for Biomedical Applications. Magnetochemistry. 2022; 8(9):107. https://doi.org/10.3390/magnetochemistry8090107
Chicago/Turabian StyleMittal, Ashi, Indrajit Roy, and Sona Gandhi. 2022. "Magnetic Nanoparticles: An Overview for Biomedical Applications" Magnetochemistry 8, no. 9: 107. https://doi.org/10.3390/magnetochemistry8090107
APA StyleMittal, A., Roy, I., & Gandhi, S. (2022). Magnetic Nanoparticles: An Overview for Biomedical Applications. Magnetochemistry, 8(9), 107. https://doi.org/10.3390/magnetochemistry8090107