Sodium Citrate Electrolyte Additive to Improve Zinc Anode Behavior in Aqueous Zinc-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
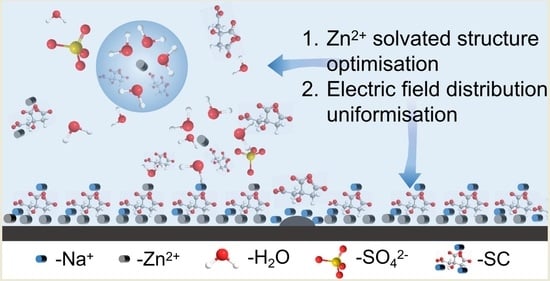
3.1. Properties and Mechanism of Electrolyte with SC Additive
3.2. Electrochemical Properties of Electrolytes with SC Additive
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, G.; Zhao, Z.; Zhang, S.; Sun, L.; Li, M.; Yuwono, J.A.; Mao, J.; Hao, J.; Vongsvivut, J.; Xing, L.; et al. A biocompatible electrolyte enables highly reversible Zn anode for zinc ion battery. Nat. Commun. 2023, 14, 6526. [Google Scholar] [CrossRef]
- Yang, C.; Wu, Q.; Xie, W.; Zhang, X.; Brozena, A.; Zheng, J.; Garaga, M.N.; Ko, B.H.; Mao, Y.; He, S.; et al. Copper-coordinated cellulose ion conductors for solid-state batteries. Nature 2021, 598, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.S.; Srinivasan, V.; Xu, K. Designing better electrolytes. Science 2022, 378, eabq3750. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Ryu, J.-H.; Deepika; Lim, Y.R.; Nah, I.W.; Lee, K.-R.; Archer, L.A.; Il Cho, W. Langmuir–Blodgett artificial solid-electrolyte interphases for practical lithium metal batteries. Nat. Energy 2018, 3, 889–898. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Q.; Tang, T.; Yin, J.; Quilty, C.D.; Renderos, G.D.; Liu, X.; Deng, Y.; Wang, L.; Bock, D.C.; et al. Reversible epitaxial electrodeposition of metals in battery anodes. Science 2019, 366, 645–648. [Google Scholar] [CrossRef]
- Liu, A.N.; Wu, F.; Zhang, Y.X.; Jiang, Y.; Xie, C.; Yang, K.Q.; Zhou, J.H.; Xie, M. Ultralarge layer spacing and superior structural stability of V2O5 as high-performance cathode for aqueous zinc-ion battery. Nano Res. 2023, 16, 9461–9470. [Google Scholar] [CrossRef]
- Sun, G.Q.; Zhou, M.Q.; Dong, X.Y.; Zang, S.Q.; Qu, L.T. An efficient and versatile biopolishing strategy to construct high performance zinc anode. Nano Res. 2022, 15, 5081–5088. [Google Scholar] [CrossRef]
- Xia, X.Y.; Zhao, Y.J.; Zhao, Y.; Xu, M.G.; Liu, W.; Sun, X.M. Mo doping provokes two electron reaction in MnO2 with ultrahigh capacity for aqueous zinc ion batteries. Nano Res. 2023, 16, 2511–2518. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry With H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, Y.; Zhao, Q.; Lei, K.; Chen, C.; Liu, X.; Chen, J. Cation-Deficient Spinel ZnMn2O4 Cathode in Zn(CF3SO3)2 Electrolyte for Rechargeable Aqueous Zn-Ion Battery. J. Am. Chem. Soc. 2016, 138, 12894–12901. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Fan, G.; Liu, J.; Liu, F.; Li, J.; Zhou, X.; Ni, Y.; Yu, M.; Zhang, Y.-M.; Su, H.; et al. Boosting the Kinetics and Stability of Zn Anodes in Aqueous Electrolytes with Supramolecular Cyclodextrin Additives. J. Am. Chem. Soc. 2022, 144, 11129–11137. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Jiao, Y.; Wu, P. Guiding Zn Uniform Deposition with Polymer Additives for Long-lasting and Highly Utilized Zn Metal Anodes. Angew. Chem. Int. Ed. 2023, 62, e202314456. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 2019, 12, 1938–1949. [Google Scholar] [CrossRef]
- Zeng, L.; He, H.; Chen, H.; Luo, D.; He, J.; Zhang, C. 3D Printing Architecting Reservoir-Integrated Anode for Dendrite-Free, Safe, and Durable Zn Batteries. Adv. Energy Mater. 2022, 12, 2103708. [Google Scholar] [CrossRef]
- Xue, P.; Guo, C.; Li, L.; Li, H.; Luo, D.; Tan, L.; Chen, Z. A MOF-Derivative Decorated Hierarchical Porous Host Enabling Ultrahigh Rates and Superior Long-Term Cycling of Dendrite-Free Zn Metal Anodes. Adv. Mater. 2022, 34, 2110047. [Google Scholar] [CrossRef] [PubMed]
- Joseph, F.; Parker; Chervin, C.N.; Pala, I.R.; Machler, M.; Burz, M.F.; Long, J.W.; Rolison, D.R. Rechargeable nickel–3D zinc batteries: An energy-dense, safer alternative to lithium-ion. Science 2017, 356, 415–418. [Google Scholar]
- Zhao, N.; Liang, Y.; Huo, W.; Zhu, X.; He, Z.; Zhang, Z.; Zhang, Y.; Wu, X.; Dai, L.; Zhu, J.; et al. Separator functionalization enables high-performance zinc anode via ion-migration regulation and interfacial engineering. Chin. Chem. Lett. 2023, 109332. [Google Scholar] [CrossRef]
- Shao, W.; Li, C.; Wang, C.; Du, G.; Zhao, S.; Qu, G.; Xing, Y.; Guo, T.; Li, H.; Xu, X. Stabilization of zinc anode by trace organic corrosion inhibitors for long lifespan. Chin. Chem. Lett. 2024, 109531. [Google Scholar] [CrossRef]
- Hao, J.; Long, J.; Li, B.; Li, X.; Zhang, S.; Yang, F.; Zeng, X.; Yang, Z.; Pang, W.K.; Guo, Z. Toward High-Performance Hybrid Zn-Based Batteries via Deeply Understanding Their Mechanism and Using Electrolyte Additive. Adv. Funct. Mater. 2019, 29, 1903605. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z.; Li, J.; Luo, N.; Chai, G.-L.; Miller, T.S.; Lai, F.; Shearing, P.; Brett, D.J.L.; Han, D.; et al. Alleviation of Dendrite Formation on Zinc Anodes via Electrolyte Additives. ACS Energy Lett. 2021, 6, 395–403. [Google Scholar] [CrossRef]
- Chen, W.; Guo, S.; Qin, L.; Li, L.; Cao, X.; Zhou, J.; Luo, Z.; Fang, G.; Liang, S. Hydrogen Bond-Functionalized Massive Solvation Modules Stabilizing Bilateral Interfaces. Adv. Funct. Mater. 2022, 32, 2112609. [Google Scholar] [CrossRef]
- Wang, P.; Xie, X.; Xing, Z.; Chen, X.; Fang, G.; Lu, B.; Zhou, J.; Liang, S.; Fan, H.J. Mechanistic Insights of Mg2+-Electrolyte Additive for High-Energy and Long-Life Zinc-Ion Hybrid Capacitors. Adv. Energy Mater. 2021, 11, 2101158. [Google Scholar] [CrossRef]
- Zeng, X.; Qian, S.; Zhou, J.; Hao, B.; Zhang, L.; Zhou, Y.; Shi, Y.; Zhu, C.; Zhou, X.; Liu, J.; et al. Sustained-Compensated Interfacial Zincophilic Sites to Assist High-Capacity Aqueous Zn Metal Batteries. Nano Lett. 2023, 23, 1135–1143. [Google Scholar] [CrossRef]
- Yoo, G.; Lee, Y.-G.; Im, B.; Kim, D.G.; Jo, Y.-R.; An, G.H. Integrated solution for a stable and high-performance zinc-ion battery using an electrolyte additive. Energy Storage Mater. 2023, 61, 102845. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tang, Z.; Sun, D.; Li, R.; Yang, W.; Zhou, S.; Xie, Z.; Tang, Y.; Wang, H. Sodium citrate as a self-sacrificial sodium compensation additive for sodium-ion batteries. Chem. Commun. 2021, 57, 4243–4246. [Google Scholar] [CrossRef]
- Wang, B.; Zheng, R.; Yang, W.; Han, X.; Hou, C.; Zhang, Q.; Li, Y.; Li, K.; Wang, H. Synergistic Solvation and Interface Regulations of Eco-Friendly Silk Peptide Additive Enabling Stable Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2112693. [Google Scholar] [CrossRef]
- Li, T.C.; Lim, Y.; Li, X.L.; Luo, S.; Lin, C.; Fang, D.; Xia, S.; Wang, Y.; Yang, H.Y. A Universal Additive Strategy to Reshape Electrolyte Solvation Structure toward Reversible Zn Storage. Adv. Energy Mater. 2022, 12, 2103231. [Google Scholar] [CrossRef]
- Feng, D.; Cao, F.; Hou, L.; Li, T.; Jiao, Y.; Wu, P. Immunizing Aqueous Zn Batteries against Dendrite Formation and Side Reactions at Various Temperatures via Electrolyte Additives. Small 2021, 17, 2103195. [Google Scholar] [CrossRef]
- Lee, C.-J.; Wu, H.; Hu, Y.; Young, M.; Wang, H.; Lynch, D.; Xu, F.; Cong, H.; Cheng, G. Ionic Conductivity of Polyelectrolyte Hydrogels. Acs Appl. Mater. Inter. 2018, 10, 5845–5852. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, S.; Wu, S.; Hu, Z.; Cui, G.; Luo, J. In situ built interphase with high interface energy and fast kinetics for high performance Zn metal anodes. Energy Environ. Sci. 2021, 14, 3609–3620. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Q.; Li, F.; Chen, J.; Yuan, H.; Li, Y.; Hu, P.; Kurbanov, M.S.; Wang, H. Low-temperature and high-rate Zn metal batteries enabled by mitigating Zn2+ concentration polarization. Chem. Eng. J. 2022, 433, 134589. [Google Scholar] [CrossRef]
- Yang, H.; Qiao, Y.; Chang, Z.; Deng, H.; Zhu, X.; Zhu, R.; Xiong, Z.; He, P.; Zhou, H. Reducing Water Activity by Zeolite Molecular Sieve Membrane for Long-Life Rechargeable Zinc Battery. Adv. Mater. 2021, 33, 2102415. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, F.; Deng, W.; Zhang, H.; Wang, X. Dual-function electrolyte additive enabling simultaneous electrode interface and coordination environment regulation for zinc-ion batteries. Energy Storage Mater. 2023, 58, 20–29. [Google Scholar] [CrossRef]
- Shangguan, M.; Wang, K.; Zhao, Y.; Xia, L. Tetraethylene Glycol Dimethyl Ether (TEGDME)-Water Hybrid Electrolytes Enable Excellent Cyclability in Aqueous Zn-Ion Batteries. Batteries 2023, 9, 462. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Graff, G.L.; Zhang, J.; Sushko, M.L.; Chen, X.; Shao, Y.; Engelhard, M.H.; Nie, Z.; Xiao, J.; et al. Dendrite-Free Lithium Deposition via Self-Healing Electrostatic Shield Mechanism. J. Am. Chem. Soc. 2013, 135, 4450–4456. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Pan, L.; Peng, Z.; Sun, Z.; Lin, H.; Mao, C.; Wang, L.; Dai, L.; Liu, H.; Pan, K.; et al. Electrolyte additive engineering for aqueous Zn ion batteries. Energy Storage Mater. 2022, 51, 733–755. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, K.; Li, X.; Yan, C.; Liu, Q.; Tang, A. Tuning the ferrous coordination structure enables a highly reversible Fe anode for long-life all-iron flow batteries. J. Mater. Chem. A 2021, 9, 26354–26361. [Google Scholar] [CrossRef]
- Qiu, K.; Trudgeon, D.; Li, X.; Yufit, V.; Chakrabarti, B.; Brandon, N.; Shah, A. Study of Quaternary Ammonium Additives towards High-Rate Zinc Deposition and Dissolution Cycling for Application in Zinc-Based Rechargeable Batteries. Batteries 2022, 8, 106. [Google Scholar] [CrossRef]
- Yu, H.; Chen, D.; Li, Q.; Yan, C.; Jiang, Z.; Zhou, L.; Wei, W.; Ma, J.; Ji, X.; Chen, Y.; et al. In Situ Construction of Anode–Molecule Interface via Lone-Pair Electrons in Trace Organic Molecules Additives to Achieve Stable Zinc Metal Anodes. Adv. Energy Mater. 2023, 13, 2300550. [Google Scholar] [CrossRef]
- He, J.; Tang, Y.; Liu, G.; Li, H.; Ye, M.; Zhang, Y.; Yang, Q.; Liu, X.; Li, C. Intrinsic Hydrogen-Bond Donors-Lined Organophosphate Superionic Nanochannels Levering High-Rate-Endurable Aqueous Zn Batteries. Adv. Energy Mater. 2022, 12, 2202661. [Google Scholar] [CrossRef]





| Cation | Effective Reduction Potential () | |||||
|---|---|---|---|---|---|---|
| 1.0 M | 1.5 mM | 3.0 mM | 15 mM | 30 mM | 2.0 M | |
| Zn2+ | −0.762 | −0.846 | −0.837 | −0.816 | −0.807 | −0.753 |
| Na+ | −2.71 | −2.877 | −2.859 | −2.818 | −2.800 | −2.692 |
| Sample | Thickness | R0 | Area | Ionic Conductivity |
|---|---|---|---|---|
| μm | Ω | cm−2 | mS cm−1 | |
| ZSO-0.5 SC | 276 | 3.3 | 0.785 | 10.65 |
| ZSO-1.0 SC | 276 | 3.1 | 0.785 | 11.34 |
| ZSO-5.0 SC | 276 | 2.8 | 0.785 | 12.56 |
| ZSO-10 SC | 276 | 3.9 | 0.785 | 9.02 |
| ZSO | 276 | 4.2 | 0.785 | 8.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yue, L.; Dong, W.; Qu, Y.; Sun, X.; Chen, L. Sodium Citrate Electrolyte Additive to Improve Zinc Anode Behavior in Aqueous Zinc-Ion Batteries. Batteries 2024, 10, 97. https://doi.org/10.3390/batteries10030097
Liu X, Yue L, Dong W, Qu Y, Sun X, Chen L. Sodium Citrate Electrolyte Additive to Improve Zinc Anode Behavior in Aqueous Zinc-Ion Batteries. Batteries. 2024; 10(3):97. https://doi.org/10.3390/batteries10030097
Chicago/Turabian StyleLiu, Xin, Liang Yue, Weixu Dong, Yifan Qu, Xianzhong Sun, and Lifeng Chen. 2024. "Sodium Citrate Electrolyte Additive to Improve Zinc Anode Behavior in Aqueous Zinc-Ion Batteries" Batteries 10, no. 3: 97. https://doi.org/10.3390/batteries10030097
APA StyleLiu, X., Yue, L., Dong, W., Qu, Y., Sun, X., & Chen, L. (2024). Sodium Citrate Electrolyte Additive to Improve Zinc Anode Behavior in Aqueous Zinc-Ion Batteries. Batteries, 10(3), 97. https://doi.org/10.3390/batteries10030097