Flexible and Lightweight Lithium-Ion Batteries Based on Cellulose Nanofibrils and Carbon Fibers
Abstract
:1. Introduction
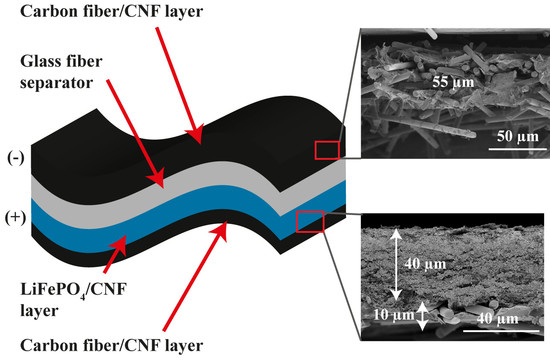
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Flexible CF Electrodes and LFP-CF Electrodes
3.3. Material Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, S.; Kwon, H.J.; Lee, S.; Shim, H.; Chun, Y.; Choi, W.; Kwack, J.; Han, D.; Song, M.; Kim, S. Low-Power Flexible Organic Light-Emitting Diode Display Device. Adv. Mater. 2011, 23, 3511–3516. [Google Scholar] [CrossRef] [PubMed]
- Abad, E.; Zampolli, S.; Marco, S.; Scorzoni, A.; Mazzolai, B.; Juarros, A.; Gómez, D.; Elmi, I.; Cardinali, G.C.; Gómez, J.M. Flexible Tag Microlab Development: Gas Sensors Integration in RFID Flexible Tags for Food Logistic. Sens. Actuators B 2007, 127, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Khan, Y.; Garg, M.; Gui, Q.; Schadt, M.; Gaikwad, A.; Han, D.; Yamamoto, N.A.; Hart, P.; Welte, R.; Wilson, W. Flexible Hybrid Electronics: Direct Interfacing of Soft and Hard Electronics for Wearable Health Monitoring. Adv. Funct. Mater. 2016, 26, 8764–8775. [Google Scholar] [CrossRef]
- Pang, C.; Lee, C.; Suh, K.Y. Recent Advances in Flexible Sensors for Wearable and Implantable Devices. J. Appl. Polym. Sci. 2013, 130, 1429–1441. [Google Scholar] [CrossRef]
- Hu, L.B.; Wu, H.; La Mantia, F.; Yang, Y.A.; Cui, Y. Thin, Flexible Secondary Li-Ion Paper Batteries. ACS Nano 2010, 4, 5843–5848. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, L.; Nyström, G.; Mihranyan, A.; Strømme, M. Toward Flexible Polymer and Paper-Based Energy Storage Devices. Adv. Mater. 2011, 23, 3751–3769. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, C.; Tammela, P.; Huo, J.; Stromme, M.; Edström, K.; Gustafsson, T.; Nyholm, L. Flexible Freestanding Cladophora Nanocellulose Paper Based Si Anodes for Lithium-Ion Batteries. J. Mater. Chem. A 2015, 3, 14109–14115. [Google Scholar] [CrossRef]
- Choi, K.H.; Cho, S.J.; Kim, S.H.; Kwon, Y.H.; Kim, J.Y.; Lee, S.Y. Thin, Deformable, and Safety-Reinforced Plastic Crystal Polymer Electrolytes for High-Performance Flexible Lithium-Ion Batteries. Adv. Funct. Mater. 2014, 24, 44–52. [Google Scholar] [CrossRef]
- Jabbour, L.; Gerbaldi, C.; Chaussy, D.; Zeno, E.; Bodoardo, S.; Beneventi, D. Microfibrillated Cellulose–Graphite Nanocomposites for Highly Flexible Paper-Like Li-Ion Battery Electrodes. J. Mater. Chem. 2010, 20, 7344–7347. [Google Scholar] [CrossRef]
- Jabbour, L.; Chaussy, D.; Eyraud, B.; Beneventi, D. Highly Conductive Graphite/Carbon Fiber/Cellulose Composite Papers. Compos. Sci. Technol. 2012, 72, 616–623. [Google Scholar] [CrossRef]
- Jabbour, L.; Destro, M.; Gerbaldi, C.; Chaussy, D.; Penazzi, N.; Beneventi, D. Aqueous Processing of Cellulose Based Paper-Anodes for Flexible Li-Ion Batteries. J. Mater. Chem. 2012, 22, 3227–3233. [Google Scholar] [CrossRef]
- Jabbour, L.; Destro, M.; Chaussy, D.; Gerbaldi, C.; Penazzi, N.; Bodoardo, S.; Beneventi, D. Flexible Cellulose/LiFePO4 Paper-Cathodes: Toward Eco-Friendly All-Paper Li-Ion Batteries. Cellulose 2013, 20, 571–582. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, X.; Zhang, X.; Yang, L.; Zhang, X.; Chen, H.; Yang, M.; Fang, D. Free-Standing and Flexible Limntio 4/Carbon Nanotube Cathodes for High Performance Lithium Ion Batteries. J. Power Sources 2016, 321, 120–125. [Google Scholar] [CrossRef]
- Wang, K.; Luo, S.; Wu, Y.; He, X.; Zhao, F.; Wang, J.; Jiang, K.; Fan, S. Super-Aligned Carbon Nanotube Films as Current Collectors for Lightweight and Flexible Lithium Ion Batteries. Adv. Funct. Mater. 2013, 23, 846–853. [Google Scholar] [CrossRef]
- Chu, H.-C.; Tuan, H.-Y. High-Performance Lithium-Ion Batteries with 1.5 Μm Thin Copper Nanowire Foil as a Current Collector. J. Power Sources 2017, 346, 40–48. [Google Scholar] [CrossRef]
- Li, N.; Chen, Z.; Ren, W.; Li, F.; Cheng, H.-M. Flexible Graphene-Based Lithium Ion Batteries with Ultrafast Charge and Discharge Rates. Proc. Natl. Acad. Sci. USA 2012, 109, 17360–17365. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, S.; Kim, S.; Park, K.-W.; Cho, D.; Jeong, Y. Carbon Nanotube Film Anodes for Flexible Lithium Ion Batteries. J. Power Sources 2015, 279, 495–501. [Google Scholar] [CrossRef]
- Martha, S.K.; Dudney, N.J.; Kiggans, J.O.; Nanda, J. Electrochemical Stability of Carbon Fibers Compared to Aluminum as Current Collectors for Lithium-Ion Batteries. J. Electrochem. Soc. 2012, 159, A1652–A1658. [Google Scholar] [CrossRef]
- Jabbour, L.; Destro, M.; Chaussy, D.; Gerbaldi, C.; Bodoardo, S.; Penazzi, N.; Beneventi, D. Cellulose/Graphite/Carbon Fibres Composite Electrodes for Li-Ion Batteries. Compos. Sci. Technol. 2013, 87, 232–239. [Google Scholar] [CrossRef]
- Martha, S.K.; Kiggans, J.O.; Nanda, J.; Dudney, N.J. Advanced Lithium Battery Cathodes Using Dispersed Carbon Fibers as the Current Collector. J. Electrochem. Soc. 2011, 158, A1060–A1066. [Google Scholar] [CrossRef]
- Lee, J.K.; An, K.W.; Ju, J.B.; Cho, B.W.; Cho, W.I.; Park, D.; Yun, K.S. Electrochemical Properties of Pan-Based Carbon Fibers as Anodes for Rechargeable Lithium Ion Batteries. Carbon 2001, 39, 1299–1305. [Google Scholar] [CrossRef]
- Kjell, M.H.; Jacques, E.; Zenkert, D.; Behm, M.; Lindbergh, G. Pan-Based Carbon Fiber Negative Electrodes for Structural Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, A1455–A1460. [Google Scholar] [CrossRef]
- Hagberg, J.; Leijonmarck, S.; Lindbergh, G. High Precision Coulometry of Commercial Pan-Based Carbon Fibers as Electrodes in Structural Batteries. J. Electrochem. Soc. 2016, 163, A1790–A1797. [Google Scholar] [CrossRef]
- Lu, H.; Behm, M.; Leijonmarck, S.; Lindbergh, G.; Cornell, A. Flexible Paper Electrodes for Li-Ion Batteries Using Low Amount of TEMPO-Oxidized Cellulose Nanofibrils as Binder. ACS Appl. Mater. Interfaces 2016, 8, 18097–18106. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Feng, X.; Song, Y.; Liu, H.; Miao, M.; Fang, J.; Shi, L. In-Situ Carbonized Cellulose-Based Hybrid Film as Flexible Paper-Anode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Z.-H.; Ma, W.-J.; Ma, Q.-S. Influence of Heat Treatment on Physical–Chemical Properties of Pan-Based Carbon Fiber. Ceram. Int. 2006, 32, 291–295. [Google Scholar] [CrossRef]
- Stevens, D.; Dahn, J. The Mechanisms of Lithium and Sodium Insertion in Carbon Materials. J. Electrochem. Soc. 2001, 148, A803–A811. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of Nano-Sized Plant Cellulose Fibrils by Direct Surface Carboxylation Using TEMPO Catalyst under Neutral Conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Leijonmarck, S.; Cornell, A.; Lindbergh, G.; Wågberg, L. Flexible Nano-Paper-Based Positive Electrodes for Li-Ion Batteries—Preparation Process and Properties. Nano Energy 2013, 2, 794–800. [Google Scholar] [CrossRef]
- Leijonmarck, S.; Cornell, A.; Lindbergh, G.; Wågberg, L. Single-Paper Flexible Li-Ion Battery Cells through a Paper-Making Process Based on Nano-Fibrillated Cellulose. J. Mater. Chem. A 2013, 1, 4671–4677. [Google Scholar] [CrossRef]







| BET Surface Area before Chopping (m2 g−1) | BET Surface Area after Chopping (m2 g−1) | d002 (Å) | Lc (Å) |
|---|---|---|---|
| 0.42 | 0.65 | 3.50 | 24.3 |
| Electrode | Young’s Modulus (MPa) | Ultimate Strength (MPa) | Strain at Break (%) | Conductivity (S m−1) |
|---|---|---|---|---|
| LFP-CF | 201 ± 12.7 | 1.97 ± 0.02 | 2.06 ± 0.29 | 95 |
| CF/SPC/CNF | 225 ± 10.7 | 1.91 ± 0.04 | 1.52 ± 0.12 | 143 |
| Function | Layer | LFP (wt%) | CF (wt%) | SPC (wt%) | CNF (wt%) |
|---|---|---|---|---|---|
| Negative electrode | CF/CNF | - | 96 | - | 4 |
| Negative electrode | CF/SPC/CNF | - | 94 | 2 | 4 |
| Positive electrode | LFP/SPC/CNF | 85 | - | 11 | 4 |
| Current collector for positive electrode | CF/CNF | - | 96 | - | 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Hagberg, J.; Lindbergh, G.; Cornell, A. Flexible and Lightweight Lithium-Ion Batteries Based on Cellulose Nanofibrils and Carbon Fibers. Batteries 2018, 4, 17. https://doi.org/10.3390/batteries4020017
Lu H, Hagberg J, Lindbergh G, Cornell A. Flexible and Lightweight Lithium-Ion Batteries Based on Cellulose Nanofibrils and Carbon Fibers. Batteries. 2018; 4(2):17. https://doi.org/10.3390/batteries4020017
Chicago/Turabian StyleLu, Huiran, Johan Hagberg, Göran Lindbergh, and Ann Cornell. 2018. "Flexible and Lightweight Lithium-Ion Batteries Based on Cellulose Nanofibrils and Carbon Fibers" Batteries 4, no. 2: 17. https://doi.org/10.3390/batteries4020017
APA StyleLu, H., Hagberg, J., Lindbergh, G., & Cornell, A. (2018). Flexible and Lightweight Lithium-Ion Batteries Based on Cellulose Nanofibrils and Carbon Fibers. Batteries, 4(2), 17. https://doi.org/10.3390/batteries4020017