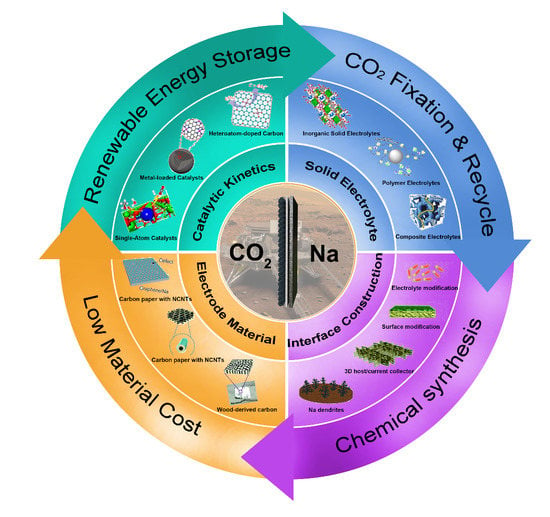
Recent Progress and Perspectives of Solid State Na-CO2 Batteries
Abstract
:1. Introduction
2. Mechanism of Na-CO2 Electrochemistry
3. Cathode Material/Catalysts of Na-CO2 Batteries
3.1. Carbon Materials and Heteroatom-Doped Carbon Materials
3.1.1. Commercial Carbon Materials
3.1.2. Nanocarbon Materials
3.1.3. Heteroatom-Doped Carbon Materials

3.2. Metal-Loaded Composites
3.2.1. Precious Metals and Their Composites
3.2.2. Transition Metals and Their Composites

3.2.3. Other Types of Composite Materials
3.3. Single-Atom Catalysts
4. Progress of Solid Electrolyte and Interface Research
4.1. Inorganic Solid Electrolytes
4.1.1. NASICON Structured Electrolytes
4.1.2. Na-Beta-Al2O3
4.1.3. Sulfide Electrolytes
4.1.4. Complex Hydride Electrolytes

4.2. Polymer Electrolytes
4.2.1. Solvent-Free Polymer Electrolytes
4.2.2. Gel Polymer Electrolytes
4.3. Composite Polymer Electrolytes

4.4. Interfaces of Solid Electrolytes

5. In Situ Characterization Techniques and Theoretical Calculations
5.1. In Situ Characterization Techniques
5.1.1. In Situ Diffraction Technique
5.1.2. In Situ Microscopy Techniques


5.1.3. In Situ Spectroscopy Technique
5.2. Theoretical Calculations and Simulations
5.2.1. Theoretical Calculation and Simulation Methods Based on Quantum Mechanics
5.2.2. Introduction to Material Genetic Engineering and Machine Learning
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xie, J.F.; Zhou, Z.; Wang, Y.B. Metal-CO2 Batteries at the Crossroad to Practical Energy Storage and CO2 Recycle. Adv. Funct. Mater. 2020, 30, 1908285. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Xia, X.H.; Cai, W.; Chen, Q.G.; Chen, M.H. Metal-CO2 Electrochemistry: From CO2 Recycling to Energy Storage. Adv. Energy Mater. 2021, 11, 2100667. [Google Scholar] [CrossRef]
- Xia, C.; Black, R.; Fernandes, R.; Adams, B.; Nazar, L.F. The critical role of phase-transfer catalysis in aprotic sodium oxygen batteries. Nat. Chem. 2015, 7, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Li, W.J.; Han, C.; Wang, W.L.; Gebert, F.; Chou, S.L.; Liu, H.K.; Zhang, X.H.; Dou, S.X. Commercial Prospects of Existing Cathode Materials for Sodium Ion Storage. Adv. Energy Mater. 2017, 7, 1700274. [Google Scholar] [CrossRef]
- Hu, X.F.; Sun, J.C.; Li, Z.F.; Zhao, Q.; Chen, C.C.; Chen, J. Rechargeable Room-Temperature Na-CO2 Batteries. Angew. Chem. Int. Ed. 2016, 55, 6482–6486. [Google Scholar] [CrossRef]
- Jena, A.; Tong, Z.Z.; Chang, H.; Hu, S.F.; Liu, R.S. Capturing carbon dioxide in Na-CO2 batteries: A route for green energy. J. Chin. Chem. Soc. 2020, 68, 421–428. [Google Scholar] [CrossRef]
- Pham, T.A.; Kweon, K.E.; Samanta, A.; Lordi, V.; Pask, J.E. Solvation and Dynamics of Sodium and Potassium in Ethylene Carbonate from ab Initio Molecular Dynamics Simulations. J. Phys. Chem. C 2017, 121, 21913–21920. [Google Scholar] [CrossRef]
- Mu, X.W.; Pan, H.; He, P.; Zhou, H.S. Li-CO2 and Na-CO2 Batteries: Toward Greener and Sustainable Electrical Energy Storage. Adv. Mater. 2020, 37, e1903790. [Google Scholar]
- Xie, Z.J.; Zhang, X.; Zhang, Z.; Zhou, Z. Metal-CO2 Batteries on the Road: CO2 from Contamination Gas to Energy Source. Adv. Mater. 2017, 29, 1605891. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, C.; Gu, Q.; Konstantinov, K.; Wang, J. Research Progress and Future Perspectives on Rechargeable Na-O2 and Na-CO2 Batteries. Energy Environ. Mater. 2021, 4, 158–177. [Google Scholar] [CrossRef]
- Takechi, K.; Shiga, T.; Asaoka, T. A Li-O2/CO2 battery. Chem. Commun. 2011, 47, 3463–3465. [Google Scholar] [CrossRef]
- Das, S.K.; Xu, S.M.; Archer, L.A. Carbon dioxide assist for non-aqueous sodium-oxygen batteries. Electrochem. Commun. 2013, 27, 59–62. [Google Scholar] [CrossRef]
- Liu, B.; Sun, Y.L.; Liu, L.Y.; Chen, J.T.; Yang, B.J.; Xu, S.; Yan, X.B. Recent advances in understanding Li-CO2 electrochemistry. Energy Environ. Sci. 2019, 12, 887–922. [Google Scholar] [CrossRef]
- Xu, S.M.; Lu, Y.Y.; Wang, H.S.; Abruña, H.D.; Archer, L.A. A rechargeable Na-CO2/O2 battery enabled by stable nanoparticle hybrid electrolytes. J. Mater. Chem. A 2014, 2, 17723–17729. [Google Scholar] [CrossRef]
- Guo, L.N.; Li, B.; Thirumal, V.; Song, J.X. Advanced rechargeable Na-CO2 batteries enabled by a ruthenium@porous carbon composite cathode with enhanced Na2CO3 reversibility. Chem. Commun. 2019, 55, 7946–7949. [Google Scholar] [CrossRef]
- Sui, D.; Chang, M.J.; Wang, H.Y.; Qian, H.; Yang, Y.L.; Li, S.; Zhang, Y.S.; Song, Y.Z. A Brief Review of Catalytic Cathode Materials for Na-CO2 Batteries. Catalysts 2021, 11, 603. [Google Scholar] [CrossRef]
- Zhou, J.W.; Li, X.L.; Yang, C.; Li, Y.C.; Guo, K.K.; Cheng, J.L.; Yuan, D.W.; Song, C.H.; Lu, J.; Wang, B. A Quasi-Solid-State Flexible Fiber-Shaped Li-CO2 Battery with Low Overpotential and High Energy Efficiency. Adv. Mater. 2019, 31, e1804439. [Google Scholar] [CrossRef]
- Yang, C.; Guo, K.K.; Yuan, D.W.; Cheng, J.L.; Wang, B. Unraveling Reaction Mechanisms of Mo2C as Cathode Catalyst in a Li-CO2 Battery. J. Am. Chem. Soc. 2020, 142, 6983–6990. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Chen, Y.N.; Bao, J.; Zhou, X.L.; Xie, Z.J.; Wei, J.P.; Zhou, Z. The First Introduction of Graphene to Rechargeable Li-CO2 Batteries. Angew. Chem. 2015, 127, 6550–6653. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.G.; Zhang, X.; Xie, Z.J.; Chen, Y.N.; Ma, L.P.; Peng, Z.Q.; Zhou, Z. Verifying the Rechargeability of Li-CO2 Batteries on Working Cathodes of Ni Nanoparticles Highly Dispersed on N-Doped Graphene. Adv. Sci. 2017, 5, 1700567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Zhang, Z.; Chen, Y.N.; Xie, Z.J.; Wei, J.P.; Zhou, Z. Rechargeable Li-CO2 batteries with carbon nanotubes as air cathodes. Chem. Commun. 2015, 51, 14636–14639. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Zhao, N.; Li, Y.Q.; Guo, X.X.; Feng, X.F.; Liu, X.S.; Liu, Z.; Cui, G.L.; Zheng, H.; Gu, L.; et al. A Rechargeable Li-Air Fuel Cell Battery Based on Garnet Solid Electrolytes. Sci. Rep. 2017, 7, 41217. [Google Scholar] [CrossRef] [Green Version]
- Li, S.W.; Dong, Y.; Zhou, J.W.; Liu, Y.; Wang, J.M.; Gao, X.; Han, Y.Z.; Qi, P.F.; Wang, B. Carbon dioxide in the cage: Manganese metal–organic frameworks for high performance CO2 electrodes in Li-CO2 batteries. Energy Environ. Sci. 2018, 11, 1318–1325. [Google Scholar] [CrossRef]
- Hu, X.F.; Joo, P.H.; Matios, E.; Wang, C.L.; Luo, J.M.; Yang, K.S.; Li, W.Y. Designing an All-Solid-State Sodium-Carbon Dioxide Battery Enabled by Nitrogen-Doped Nanocarbon. Nano Lett. 2020, 20, 3620–3626. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.M.; Das, S.K.; Archer, L.A. The Li-CO2 battery: A novel method for CO2 capture and utilization. RSC Adv. 2013, 3, 6656–6660. [Google Scholar] [CrossRef]
- Yang, S.X.; Qiao, Y.; He, P.; Liu, Y.J.; Cheng, Z.; Zhu, J.J.; Zhou, H.S. A reversible lithium-CO2 battery with Ru nanoparticles as a cathode catalyst. Energy Environ. Sci. 2017, 10, 972–978. [Google Scholar] [CrossRef]
- Ha, T.A.; Pozo-Gonzalo, C.; Nairn, K.; MacFarlane, D.R.; Forsyth, M.; Howlett, P.C. An investigation of commercial carbon air cathode structure in ionic liquid based sodium oxygen batteries. Sci. Rep. 2020, 10, 7123. [Google Scholar] [CrossRef]
- Lu, Y.; Cai, Y.C.; Zhang, Q.; Liu, L.J.; Niu, Z.Q.; Chen, J. A compatible anode/succinonitrile-based electrolyte interface in all-solid-state Na-CO2 batteries. Chem. Sci. 2019, 10, 4306–4312. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.C.; Zhang, X.J.; Lu, Y.; Yan, Z.H.; Tao, Z.L.; Jia, D.Z.; Chen, J. Flexible and Tailorable Na-CO2 Batteries Based on an All-Solid-State Polymer Electrolyte. ChemElectroChem 2018, 5, 3628–3632. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, J.J.; Li, X.F.; Geng, D.S.; Banis, M.N.; Li, R.Y.; Sun, X.L. Nitrogen-doped graphene nanosheets as cathode materials with excellent electrocatalytic activity for high capacity lithium-oxygen batteries. Electrochem. Commun. 2012, 18, 12–15. [Google Scholar] [CrossRef]
- Ci, L.J.; Song, L.; Jin, C.H.; Jariwala, D.; Wu, D.X.; Li, Y.J.; Srivastava, A.; Wang, Z.F.; Storr, K.; Balicas, L.; et al. Atomic layers of hybridized boron nitride and graphene domains. Nat. Mater. 2010, 9, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Sui, D.; Xu, L.Q.; Zhang, H.T.; Sun, Z.H.; Kan, B.; Ma, Y.F.; Chen, Y.S. A 3D cross-linked graphene-based honeycomb carbon composite with excellent confinement effect of organic cathode material for lithium-ion batteries. Carbon 2020, 157, 656–662. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.H.; Liu, B.; Zhang, Y.; Liang, X.Q.; Xia, X.H. Heteroatom Doping: An Effective Way to Boost Sodium Ion Storage. Adv. Energy Mater. 2020, 10, 2000927. [Google Scholar] [CrossRef]
- Wang, H.; Jia, J.; Song, P.; Wang, Q.; Li, D.; Min, S.; Qian, C.; Wang, L.; Li, Y.; Ma, C.; et al. Efficient Electrocatalytic Reduction of CO2 by Nitrogen-Doped Nanoporous Carbon/Carbon Nanotube Membranes: A Step Towards the Electrochemical CO2 Refinery. Angew. Chem. Int. Ed. 2012, 18, 12–15. [Google Scholar]
- Xu, C.F.; Zhang, K.W.; Zhang, D.; Chang, S.L.; Liang, F.; Yan, P.F.; Yao, Y.C.; Qu, T.; Zhan, J.; Ma, W.H.; et al. Reversible hybrid sodium-CO2 batteries with low charging voltage and long-life. Nano Energy 2020, 68, 104318. [Google Scholar] [CrossRef]
- Li, S.W.; Liu, Y.; Zhou, J.W.; Hong, S.S.; Dong, Y.; Wang, J.M.; Gao, X.; Qi, P.F.; Han, Y.; Wang, B. Monodispersed MnO nanoparticles in graphene-an interconnected N-doped 3D carbon framework as a highly efficient gas cathode in Li-CO2 batteries. Energy Environ. Sci. 2019, 12, 1046–1054. [Google Scholar] [CrossRef]
- Dong, H.Y.; Jin, C.; Gao, Y.C.; Xiao, X.L.; Li, K.; Tang, P.P.; Li, X.N.; Yang, S.T. Nitrogen and sulfur co-doped three-dimensional graphene@NiO composite as cathode catalyst for the Li-O2 and Li-CO2 batteries. Mater. Res. Express 2019, 6, 115616. [Google Scholar] [CrossRef]
- Sun, Z.M.; Wang, D.; Lin, L.; Liu, Y.H.; Yuan, M.W.; Nan, C.Y.; Li, H.F.; Sun, G.B.; Yang, X.J. Ultrathin hexagonal boron nitride as a van der Waals’ force initiator activated graphene for engineering efficient non-metal electrocatalysts of Li-CO2 battery. Nano Res. 2022, 15, 1171–1177. [Google Scholar] [CrossRef]
- Chen, B.; Wang, D.S.; Tan, J.Y.; Liu, Y.Q.; Jiao, M.L.; Liu, B.L.; Zhao, N.Q.; Zou, X.L.; Zhou, G.M.; Cheng, H.M. Designing Electrophilic and Nucleophilic Dual Centers in the ReS2 Plane toward Efficient Bifunctional Catalysts for Li-CO2 Batteries. J. Am. Chem. Soc. 2022, 144, 3106–3116. [Google Scholar] [CrossRef]
- Shen, J.R.; Wu, H.T.; Sun, W.; Wu, Q.B.; Zhen, S.Y.; Wang, Z.H.; Sun, K.N. Biomass-derived hierarchically porous carbon skeletons with in situ decorated IrCo nanoparticles as high-performance cathode catalysts for Li-O2 batteries. J. Mater. Chem. A 2019, 7, 10662–10671. [Google Scholar] [CrossRef]
- Tong, Z.Z.; Wang, S.B.; Fang, M.H.; Lin, Y.T.; Tsai, K.T.; Tsai, S.Y.; Yin, L.C.; Hu, S.F.; Liu, R.S. Na-CO2 battery with NASICON-structured solid-state electrolyte. Nano Energy 2021, 85, 105972. [Google Scholar] [CrossRef]
- Thoka, S.; Tong, Z.Z.; Jena, A.; Hung, T.F.; Wu, C.C.; Chang, W.S.; Wang, F.M.; Wang, X.C.; Yin, L.C.; Chang, H.; et al. High-performance Na-CO2 batteries with ZnCo2O4@CNT as the cathode catalyst. J. Mater. Chem. A 2020, 8, 23974–23982. [Google Scholar] [CrossRef]
- Zhu, Y.; Feng, S.; Zhang, P.; Guo, M.; Wang, Q.; Wu, D.; Zhang, L.; Li, H.; Wang, H.; Chen, L.; et al. Probing the electrochemical evolutions of Na-CO2 nanobatteries on Pt@NCNT cathodes using in-situ environmental TEM. Energy Storage Mater. 2020, 33, 88–94. [Google Scholar] [CrossRef]
- Xu, C.; Zhan, J.; Wang, Z.; Fang, X.; Chen, J.; Liang, F.; Zhao, H.; Lei, Y. Biomass-derived highly dispersed Co/Co9S8 nanoparticles encapsulated in S, N-co-doped hierarchically porous carbon as an efficient catalyst for hybrid Na-CO2 batteries. Mater. Today Energy 2021, 19, 100594. [Google Scholar] [CrossRef]
- Jin, T.; Han, Q.Q.; Jiao, L.F. Binder-Free Electrodes for Advanced Sodium-Ion Batteries. Adv. Mater. 2020, 32, 1806304. [Google Scholar] [CrossRef]
- Zhai, Y.J.; Han, P.; Yun, Q.B.; Ge, Y.Y.; Zhang, X.; Chen, Y.; Zhang, H. Phase engineering of metal nanocatalysts for electrochemical CO2 reduction. eScience 2022, 2, 467–485. [Google Scholar] [CrossRef]
- Lu, J.; Lee, Y.J.; Luo, X.; Lau, K.C.; Asadi, M.; Wang, H.H.; Brombosz, S.; Wen, J.; Zhai, D.; Chen, Z.; et al. A lithium-oxygen battery based on lithium superoxide. Nature 2016, 529, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Xu, S.M.; Liu, Y.; Dai, J.Q.; Xie, H.; Yao, Y.G.; Mu, X.W.; Chen, C.J.; Kline, D.J.; Hitz, E.M.; et al. Transient, in situ synthesis of ultrafine ruthenium nanoparticles for a high-rate Li-CO2 battery. Energy Environ. Sci. 2019, 12, 1100–1107. [Google Scholar] [CrossRef]
- Zhang, S.; Wen, Z.; Rui, K.; Shen, C.; Lu, Y.; Yang, J. Graphene nanosheets loaded with Pt nanoparticles with enhanced electrochemical performance for sodium-oxygen batteries. J. Mater. Chem. A 2015, 3, 2568–2571. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, Y.; Li, D.; Luo, M.; Chen, N.; Ye, Y.; Qian, J.; Li, L.; Yang, D.; Wu, F.; et al. Crumpled Ir Nanosheets Fully Covered on Porous Carbon Nanofibers for Long-Life Rechargeable Lithium-CO2 Batteries. Adv. Mater. 2018, 30, e1803124. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, X.; Zhang, Z.; Dong, P.; Xu, M.; Peng, H.; Zeng, X.; Zhang, Y.; Liao, S. Design of ultralong-life Li-CO2 batteries with IrO2 nanoparticles highly dispersed on nitrogen-doped carbon nanotubes. J. Mater. Chem. A 2020, 8, 3763–3770. [Google Scholar] [CrossRef]
- Wang, X.; Xie, J.; Ghausi, M.A.; Lv, J.; Huang, Y.; Wu, M.; Wang, Y.; Yao, J. Rechargeable Zn-CO2 Electrochemical Cells Mimicking Two-Step Photosynthesis. Adv. Mater. 2019, 31, e1807807. [Google Scholar] [CrossRef]
- Ma, W.; Liu, X.; Li, C.; Yin, H.; Xi, W.; Liu, R.; He, G.; Zhao, X.; Luo, J.; Ding, Y. Rechargeable Al-CO2 Batteries for Reversible Utilization of CO2. Adv. Mater. 2018, 30, e1801152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, C.; Luo, J.; Jin, C.; Yuan, H.; Sheng, O.; Huang, H.; Gan, Y.; Xia, Y.; Liang, C.; Zhang, J.; et al. Enhancing Catalyzed Decomposition of Na2CO3 with Co2MnOx Nanowire-Decorated Carbon Fibers for Advanced Na-CO2 Batteries. ACS Appl. Mater. Interfaces 2018, 10, 17240–17248. [Google Scholar] [CrossRef]
- Dong, L.Z.; Zhang, Y.; Lu, Y.F.; Zhang, L.; Huang, X.; Wang, J.H.; Liu, J.; Li, S.L.; Lan, Y.Q. A well-defined dual Mn-site based Metal-organic framework to promote CO2 reduction/evolution in Li-CO2 batteries. Chem. Commun. 2021, 57, 8937–8940. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Liu, P.; Xie, Y.; Cao, K.; Zhou, Z. Identification of cathode stability in Li-CO2 batteries with Cu nanoparticles highly dispersed on N-doped graphene. J. Mater. Chem. A 2018, 6, 3218–3223. [Google Scholar] [CrossRef]
- Xu, C.; Zhan, J.; Wang, H.; Kang, Y.; Liang, F. Dense binary Fe-Cu sites promoting CO2 utilization enable highly reversible hybrid Na-CO2 batteries. J. Mater. Chem. A 2021, 9, 22114–22128. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; Sheng, T.; Lu, Y.; Yin, Z.; Li, Y.; Peng, X.; Zhou, Y.; Li, J.; Wu, Y.; et al. Synergetic Effect of Ru and NiO in the Electrocatalytic Decomposition of Li2CO3 to Enhance the Performance of a Li-CO2/O2 Battery. ACS Catalysis 2019, 10, 1640–1651. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Z.; Yu, W.; Shang, W.; Ma, Y.; Zhu, X.; Tan, P. Ultrafine Co-Doped NiO Nanoparticles Decorated on Carbon Nanotubes Improving the Electrochemical Performance and Cycling Stability of Li-CO2 Batteries. ACS Appl. Energy Mater. 2021, 4, 11858–11866. [Google Scholar] [CrossRef]
- Ge, B.; Sun, Y.; Guo, J.; Yan, X.; Fernandez, C.; Peng, Q. A Co-Doped MnO2 Catalyst for Li-O2 Batteries with Low Overpotential and Ultrahigh Cyclability. Small 2019, 15, e1902220. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Xiong, F.Y.; Zhu, S.H.; Chen, J.H.; Xie, J.; An, Q.Y. Defect engineering in molybdenum-based electrode materials for energy storage. eScience 2022, 2, 278–294. [Google Scholar] [CrossRef]
- Kwak, W.; Lau, K.; Shin, C.; Amine, K.; Curtiss, L.; Sun, Y. A Mo2C/Carbon Nanotube Composite Cathode for Lithium-Oxygen Batteries with High Energy Efficiency and Long Cycle Life. ACS Nano 2015, 9, 4129–4137. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, J.; Liu, L.; Liu, Y.; Chou, S.; Shi, D.; Liu, H.; Wu, Y.; Zhang, W.; Chen, J. Mo2C/CNT: An Efficient Catalyst for Rechargeable Li-CO2 Batteries. Adv. Funct. Mater. 2017, 27, 1700564. [Google Scholar] [CrossRef] [Green Version]
- Asadi, M.; Sayahpour, B.; Abbasi, P.; Ngo, A.T.; Karis, K.; Jokisaari, J.R.; Liu, C.; Narayanan, B.; Gerard, M.; Yasaei, P.; et al. A lithium-oxygen battery with a long cycle life in an air-like atmosphere. Nature 2018, 555, 502–506. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Senthil Kumar, S.; Anbu Kulandainathan, M. Highly selective electrochemical reduction of carbon dioxide using Cu based metal organic framework as an electrocatalyst. Electrochem. Commun. 2012, 25, 70–73. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Wu, D.; Guo, Z.; Yin, X.; Pang, Q.; Tu, B.; Zhang, L.; Wang, Y.G.; Li, Q. Metal-organic frameworks as cathode materials for Li-O2 batteries. Adv. Mater. 2014, 26, 3258–3262. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef] [Green Version]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, W.; Wu, J.; Li, Q.; Feng, Q.; Chen, Z.; Xiong, X.; Wang, D.; Lei, Y. Regulating the coordination structure of metal single atoms for efficient electrocatalytic CO2 reduction. Energy Environ. Sci. 2020, 13, 4609–4624. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, S.; Jiang, S.P.; Wang, S. Supported Single Atoms as New Class of Catalysts for Electrochemical Reduction of Carbon Dioxide. Small Methods 2019, 3, 1800440. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, S.; Johannessen, B.; Veder, J.P.; Saunders, M.; Rowles, M.R.; Cheng, M.; Liu, C.; Chisholm, M.F.; De Marco, R.; et al. Atomically Dispersed Transition Metals on Carbon Nanotubes with Ultrahigh Loading for Selective Electrochemical Carbon Dioxide Reduction. Adv. Mater. 2018, 30, e1706287. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Najam, T.; Bashir, M.S.; Peng, L.; Nazir, M.A.; Javed, M.S. Single-atom catalysts for next-generation rechargeable batteries and fuel cells. Energy Storage Mater. 2022, 45, 301–322. [Google Scholar] [CrossRef]
- Xi, J.; Sun, H.; Wang, D.; Zhang, Z.; Duan, X.; Xiao, J.; Xiao, F.; Liu, L.; Wang, S. Confined-interface-directed synthesis of Palladium single-atom catalysts on graphene/amorphous carbon. Appl. Catal. B Environ. 2018, 225, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Wu, C.; Chen, S.; Ding, S.; Xie, Y.; Wang, C.; Wang, T.; Haleem, Y.A.; ur Rehman, Z.; Sang, Y.; et al. In situ trapped high-density single metal atoms within graphene: Iron-containing hybrids as representatives for efficient oxygen reduction. Nano Res. 2018, 11, 2217–2228. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Q.; Fu, Y.; Lei, C.; Yang, B.; Li, Z.; Lei, L.; Wu, G.; Hou, Y. Carbon-Rich Nonprecious Metal Single Atom Electrocatalysts for CO2 Reduction and Hydrogen Evolution. Small Methods 2019, 3, 1900210. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, B.; Luo, M.; Ning, S.; Peng, M.; Zhao, Y.; Lu, Y.R.; Chan, T.S.; de Groot, F.M.F.; Tan, Y. Single platinum atoms embedded in nanoporous cobalt selenide as electrocatalyst for accelerating hydrogen evolution reaction. Nat. Commun. 2019, 10, 1743. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom Catalysis Using Pt/Graphene Achieved through Atomic Layer Deposition. Sci. Rep. 2013, 3, 1775. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jia, S.; Yang, M.; Tang, Y.; Wen, Y.; Chu, S.; Wang, J.; Shan, B.; Chen, R. Activation of subnanometric Pt on Cu-modified CeO2 via redox-coupled atomic layer deposition for CO oxidation. Nat. Commun. 2020, 11, 4240. [Google Scholar] [CrossRef]
- Wei, H.; Liu, X.; Wang, A.; Zhang, L.; Qiao, B.; Yang, X.; Huang, Y.; Miao, S.; Liu, J.; Zhang, T. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun. 2014, 5, 5634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Chen, C.; Liu, S.; Hong, S.; Zhu, Q.; Qian, Q.; Han, B.; Zhang, J.; Zheng, L. Aqueous CO2 Reduction with High Efficiency Using alpha-Co(OH)2-Supported Atomic Ir Electrocatalysts. Angew. Chem. Int. Ed. 2019, 58, 4669–4673. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, X.; Jing, H.; Wang, L.; Wei, J.; Han, Y. Design and performance of Ag nanoparticle-modified graphene/SnAgCu lead-free solders. Mater. Sci. Eng. A 2016, 667, 87–96. [Google Scholar] [CrossRef]
- Yan, D.; Chen, J.; Jia, H. Temperature-Induced Structure Reconstruction to Prepare a Thermally Stable Single-Atom Platinum Catalyst. Angew. Chem. Int. Ed. 2020, 59, 13562–13567. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Li, Z.; Chen, W.; Xu, Q.; He, D.; Xi, D.; Zhang, Q.; Yuan, T.; Qu, Y.; et al. Solid-Diffusion Synthesis of Single-Atom Catalysts Directly from Bulk Metal for Efficient CO2 Reduction. Joule 2019, 3, 584–594. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Zhang, Q.; Guan, J. Applications of metal-organic framework-derived materials in fuel cells and metal-air batteries. Coord. Chem. Rev. 2020, 409, 213214. [Google Scholar] [CrossRef]
- Han, A.; Chen, W.; Zhang, S.; Zhang, M.; Han, Y.; Zhang, J.; Ji, S.; Zheng, L.; Wang, Y.; Gu, L.; et al. A Polymer Encapsulation Strategy to Synthesize Porous Nitrogen-Doped Carbon-Nanosphere-Supported Metal Isolated-Single-Atomic-Site Catalysts. Adv. Mater. 2018, 30, e1706508. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Liu, W.; Chang, C.; Tang, H.; Li, Z.; Chen, W.; Jia, C.; Yao, T.; Wei, S.; et al. Design of N-Coordinated Dual-Metal Sites: A Stable and Active Pt-Free Catalyst for Acidic Oxygen Reduction Reaction. J. Am. Chem. Soc. 2017, 139, 17281–17284. [Google Scholar] [CrossRef]
- Liu, J.J. Advanced Electron Microscopy of Metal-Support Interactions in Supported Metal Catalysts. ChemCatChem 2011, 3, 934–948. [Google Scholar] [CrossRef]
- Wang, X.; Pi, M.; Zhang, D.; Li, H.; Feng, J.; Chen, S.; Li, J. Insight into the Superior Electrocatalytic Performance of a Ternary Nickel Iron Poly-Phosphide Nanosheet Array: An X-ray Absorption Study. ACS Appl. Mater. Interfaces 2019, 11, 14059–14065. [Google Scholar] [CrossRef]
- Cheng, N.; Zhang, L.; Doyle-Davis, K.; Sun, X. Single-Atom Catalysts: From Design to Application. Electrochem. Energy Rev. 2019, 2, 539–573. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Wang, J.; Chu, H.; Chu, Y.; Chen, H.M. In Situ/Operando Studies for Designing Next-Generation Electrocatalysts. ACS Energy Lett. 2020, 5, 1281–1291. [Google Scholar] [CrossRef]
- Hui, Y.; Liu, Y.F.; Liu, X.L.; Wang, X.K.; Tian, H.; Waterhouse, G.I.; Kruger, P.E.; Telfer, S.G.; Ma, S.Q. Large-scale synthesis of N-doped carbon capsules supporting atomically dispersed iron for efficient oxygen reduction reaction electrocatalysis. eScience 2022, 2, 227–234. [Google Scholar]
- Rao, P.; Wu, D.X.; Wang, T.J.; Li, J.; Deng, P.L.; Chen, Q.; Shen, Y.J.; Chen, Y.; Tian, X.L. Single atomic cobalt electrocatalyst for efficient oxygen reduction reaction. eScience 2022, 2, 399–404. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Zhang, X.; Zhou, Z.; Hu, Z.P. A first-principles study on the electrochemical reaction activity of 3d transition metal single-atom catalysts in nitrogen-doped graphene: Trends and hints. eScience 2022, 2, 219–226. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Meng, X.; Alonso, J.A.; Fernandez-Diaz, M.T.; Sun, C. Effects of Fluorine Doping on Structural and Electrochemical Properties of Li6.25Ga0.25La3Zr2O12 as Electrolytes for Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2019, 11, 2042–2049. [Google Scholar] [CrossRef]
- Thangadurai, V.; Pinzaru, D.; Narayanan, S.; Baral, A.K. Fast Solid-State Li Ion Conducting Garnet-Type Structure Metal Oxides for Energy Storage. J. Phys. Chem. Lett. 2015, 6, 292–299. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Xu, C.; Hu, N.; Molenda, J.; Lu, L. Development of solid-state electrolytes for sodium-ion battery-A short review. Nano Mater. Sci. 2019, 1, 91–100. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Zhang, Q.; Niu, Z.; Chen, J. Electrolyte and Interface Engineering for Solid-State Sodium Batteries. Joule 2018, 2, 1747–1770. [Google Scholar] [CrossRef] [Green Version]
- Bachman, J.; Muy, S.; Grimaud, A.; Chang, H.; Pour, N.; Lux, S.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic Solid-State Electrolytes for Lithium Batteries: Mechanisms and Properties Governing Ion Conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Shi, J.; Chu, Y.S.; Yu, X.; Xu, K.; Ge, M.; Yan, H.; Li, W.; Gu, L.; et al. A Self-Forming Composite Electrolyte for Solid-State Sodium Battery with Ultralong Cycle Life. Adv. Energy Mater. 2017, 7, 1601196. [Google Scholar] [CrossRef]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Song, S.; Duong, H.M.; Korsunsky, A.M.; Hu, N.; Lu, L. A Na(+) Superionic Conductor for Room-Temperature Sodium Batteries. Sci. Rep. 2016, 6, 32330. [Google Scholar] [CrossRef]
- Zhu, Z.; Chu, I.-H.; Deng, Z.; Ong, S.P. Role of Na+ Interstitials and Dopants in Enhancing the Na+ Conductivity of the Cubic Na3PS4 Superionic Conductor. Chem. Mater. 2015, 27, 8318–8325. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Jung, K.; Nezafati, M.; Kim, C.S.; Kang, B. Sodium Ion Diffusion in Nasicon (Na3Zr2Si2PO12) Solid Electrolytes: Effects of Excess Sodium. ACS Appl. Mater. Interfaces 2016, 8, 27814–27824. [Google Scholar] [CrossRef]
- Ma, Q.; Guin, M.; Naqash, S.; Tsai, C.-L.; Tietz, F.; Guillon, O. Scandium-Substituted Na3Zr2(SiO4)2(PO4) Prepared by a Solution-Assisted Solid-State Reaction Method as Sodium-Ion Conductors. Chem. Mater. 2016, 28, 4821–4828. [Google Scholar] [CrossRef]
- Ruan, Y.; Song, S.; Liu, J.; Liu, P.; Cheng, B.; Song, X.; Battaglia, V. Improved structural stability and ionic conductivity of Na3Zr2Si2PO12 solid electrolyte by rare earth metal substitutions. Ceram. Int. 2017, 43, 7810–7815. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.; Kafalas, J. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Hong, Y. Crystal structures and crystal chemistry in the system Na1+xZr2SixP3−xO1. Mater. Res. Bull. 1976, 11, 173–182. [Google Scholar] [CrossRef]
- Im, E.; Ryu, J.H.; Baek, K.; Moon, G.D.; Kang, S.J. “Water-in-salt” and NASICON Electrolyte-Based Na-CO2 Battery. Energy Storage Mater. 2021, 37, 424–432. [Google Scholar] [CrossRef]
- Lu, L.; Sun, C.; Hao, J.; Wang, Z.; Mayer, S.F.; Fernández-Díaz, M.T.; Alonso, J.A.; Zou, B. A High-Performance Solid-State Na-CO2 Battery with Poly(Vinyliden Fluoride-co-Hexafluoropropylene)-Na3.2Zr1.9Mg0.1Si2PO12 Electrolyte. Energy Environ. Mater. 2022, 1–9. [Google Scholar]
- Jung, J.I.; Kim, D.; Kim, H.; Jo, Y.N.; Park, J.S.; Kim, Y. Progressive Assessment on the Decomposition Reaction of Na Superionic Conducting Ceramics. ACS Appl. Mater. Interfaces 2017, 9, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Lu, Y.; Alonso, J.A.; Lopez, C.A.; Fernandez-Diaz, M.T.; Zou, B.; Sun, C. A Monolithic Solid-State Sodium-Sulfur Battery with Al-Doped Na3.4Zr2(Si0.8P0.2O4)3 Electrolyte. ACS Appl. Mater. Interfaces 2021, 13, 42927–42934. [Google Scholar] [CrossRef]
- Shen, L.; Yang, J.; Liu, G.; Avdeev, M.; Yao, X. High ionic conductivity and dendrite-resistant NASICON solid electrolyte for all-solid-state sodium batteries. Mater. Today Energy 2021, 20, 100691. [Google Scholar] [CrossRef]
- Ihlefeld, J.F.; Gurniak, E.; Jones, B.H.; Wheeler, D.R.; Rodriguez, M.A.; McDaniel, A.H.; Dunn, B. Scaling Effects in Sodium Zirconium Silicate Phosphate (Na1+xZr2SixP3−xO12) Ion-Conducting Thin Films. J. Am. Ceram. Soc. 2016, 99, 2729–2736. [Google Scholar] [CrossRef]
- Yao, Y.; Kummer, J. Ion exchange properties of and rates of ionic diffusion in beta-alumina. J. Inorg. Nucl. Chem. 1967, 29, 2453–2475. [Google Scholar]
- Hueso, K.B.; Palomares, V.; Armand, M.; Rojo, T. Challenges and perspectives on high and intermediate-temperature sodium batteries. Nano Res. 2017, 10, 4082–4114. [Google Scholar] [CrossRef]
- Lu, X.; Xia, G.; Lemmon, J.P.; Yang, Z. Advanced Materials for sodium-beta alumina batteries: Status, challenges and perspectives. J. Power Source 2010, 195, 2431–2442. [Google Scholar] [CrossRef]
- Baffier, N.; Badot, J.; Colomban, P. Conductivity of ion rich β and β″ alumina: Sodium and potassium compounds. Mater. Res. Bull. 1981, 16, 259–265. [Google Scholar] [CrossRef]
- Wu, Y.; Zhuo, L.; Ming, J.; Yu, Y.; Zhao, F. Coating of Al2O3 on layered Li(Mn1/3Ni1/3Co1/3)O2 using CO2 as green precipitant and their improved electrochemical performance for lithium ion batteries. J. Energy Chem. 2013, 22, 468–476. [Google Scholar] [CrossRef]
- Ghadbeigi, L.; Szendrei, A.; Moreno, P.; Sparks, T.D.; Virkar, A.V. Synthesis of iron-doped Na-β’’-alumina+yttria-stabilized zirconia composite electrolytes by a vapor phase process. Solid State Ion. 2016, 290, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Virkar, A.V.; Gordon, R.S. Fracture Properties of Polycrystalline Lithia-Stabilized β’’ Alumina. J. Am. Ceram. Soc. 1977, 60, 58–61. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.X.; Wu, D.X.; Niu, Q.H.; Lu, P.S.; Ma, T.H.; Su, Y.B.; Chen, L.Q.; Li, H.; Wu, F. Stable Ni-rich layered oxide cathode for sulfide-based all-solid-state lithium battery. eScience 2022, 2, 537–545. [Google Scholar] [CrossRef]
- Hayashi, A.; Noi, K.; Sakuda, A.; Tatsumisago, M. Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat. Commun. 2012, 3, 856. [Google Scholar] [CrossRef] [Green Version]
- Shang, S.L.; Yu, Z.X.; Wang, Y.; Wang, D.H.; Liu, Z.K. Origin of Outstanding Phase and Moisture Stability in a Na3P1–xAsxS4 Superionic Conductor. ACS Appl. Mater. Interfaces 2017, 9, 16261–16269. [Google Scholar] [CrossRef]
- Tian, Y.; Shi, T.; Richards, W.D.; Li, J.; Kim, J.C.; Bo, S.-H.; Ceder, G. Compatibility issues between electrodes and electrolytes in solid-state batteries. Energy Environ. Sci. 2017, 10, 1150–1166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, K.; Mi, J.; Lu, L.; Zhao, L.; Wang, L.; Li, Y.; Zeng, H. Na3PSe4: A Novel Chalcogenide Solid Electrolyte with High Ionic Conductivity. Adv. Energy Mater. 2015, 5, 1501294. [Google Scholar] [CrossRef]
- Nishimura, S.; Tanibata, N.; Hayashi, A.; Tatsumisago, M.; Yamada, A. The crystal structure and sodium disorder of high-temperature polymorph β-Na3PS4. J. Mater. Chem. A 2017, 5, 25025–25030. [Google Scholar] [CrossRef]
- Jansen, M.; Henseler, U. Synthesis, Structure Determination, and Ionic Conductivity of Sodium Tetrathiophosphate. J. Solid State Chem. 1992, 99, 110–119. [Google Scholar] [CrossRef]
- Yu, Z.; Shang, S.L.; Seo, J.H.; Wang, D.; Luo, X.; Huang, Q.; Chen, S.; Lu, J.; Li, X.; Liu, Z.K.; et al. Exceptionally High Ionic Conductivity in Na3P0.62As0.38S4 with Improved Moisture Stability for Solid-State Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1605561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, D.; Yang, K.; Yan, X.; Wang, L.; Mi, J.; Xu, B.; Li, Y. Vacancy-Contained Tetragonal Na3SbS4 Superionic Conductor. Adv. Sci. 2016, 3, 1600089. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, N.; Shuai, Y.; Chen, K. Low-Cost Raw Materials Synthesized Na2.9375PS3.9375Cl0.0625 Solid Electrolyte. Energy Technol. 2020, 8, 2000606. [Google Scholar] [CrossRef]
- de Klerk, N.J.J.; Wagemaker, M. Diffusion Mechanism of the Sodium-Ion Solid Electrolyte Na3PS4 and Potential Improvements of Halogen Doping. Chem. Mater. 2016, 28, 3122–3130. [Google Scholar] [CrossRef] [Green Version]
- Krauskopf, T.; Pompe, C.; Kraft, M.A.; Zeier, W.G. Influence of Lattice Dynamics on Na+ Transport in the Solid Electrolyte Na3PS4–xSex. Chem. Mater. 2017, 29, 8859–8869. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Hood, Z.D.; Sahu, G.; Pandian, A.S.; Keum, J.K.; An, K.; Liang, C. An Air-Stable Na3SbS4 Superionic Conductor Prepared by a Rapid and Economic Synthetic Procedure. Angew. Chem. Int. Ed. Engl. 2016, 55, 8551–8555. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Sun, Y.; Jia, H.; Peng, L.; Zhang, Y.; Xie, J. Li4−xSbxSn1−xS4 solid solutions for air-stable solid electrolytes. J. Energy Chem. 2020, 41, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Oguchi, H.; Matsuo, M.; Kuromoto, S.; Kuwano, H.; Orimo, S. Sodium-ion conduction in complex hydrides NaAlH4 and Na3AlH6. J. Appl. Phys. 2012, 111, 036102. [Google Scholar] [CrossRef]
- Matsuo, M.; Oguchi, H.; Sato, T.; Takamura, H.; Tsuchida, E.; Ikeshoji, T.; Orimo, S.-I. Sodium and magnesium ionic conduction in complex hydrides. J. Alloys Compd. 2013, 580, S98–S101. [Google Scholar] [CrossRef]
- Udovic, T.J.; Matsuo, M.; Unemoto, A.; Verdal, N.; Stavila, V.; Skripov, A.V.; Rush, J.J.; Takamura, H.; Orimo, S. Sodium superionic conduction in Na2B12H12. Chem. Commun. 2014, 50, 3750–3752. [Google Scholar] [CrossRef] [PubMed]
- Verdal, N.; Udovic, T.J.; Stavila, V.; Tang, W.S.; Rush, J.J.; Skripov, A.V. Anion Reorientations in the Superionic Conducting Phase of Na2B12H12. J. Phys. Chem. C 2014, 118, 17483–17489. [Google Scholar] [CrossRef]
- Tang, W.S.; Yoshida, K.; Soloninin, A.V.; Skoryunov, R.V.; Babanova, O.A.; Skripov, A.V.; Dimitrievska, M.; Stavila, V.; Orimo, S.-I.; Udovic, T.J. Stabilizing Superionic-Conducting Structures via Mixed-Anion Solid Solutions of Monocarba-closo-borate Salts. ACS Energy Lett. 2016, 1, 659–664. [Google Scholar] [CrossRef]
- Tang, W.S.; Matsuo, M.; Wu, H.; Stavila, V.; Zhou, W.; Talin, A.A.; Soloninin, A.V.; Skoryunov, R.V.; Babanova, O.A.; Skripov, A.V.; et al. Liquid-Like Ionic Conduction in Solid Lithium and Sodium Monocarba-closo-Decaborates Near or at Room Temperature. Adv. Energy Mater. 2016, 6, 1502237. [Google Scholar] [CrossRef]
- Duchêne, L.; Kühnel, R.S.; Rentsch, D.; Remhof, A.; Hagemann, H.; Battaglia, C. A highly stable sodium solid-state electrolyte based on a dodeca/deca-borate equimolar mixture. Chem. Commun. 2017, 53, 4195–4198. [Google Scholar] [CrossRef] [Green Version]
- Duchêne, L.; Kühnel, R.S.; Stilp, E.; Cuervo Reyes, E.; Remhof, A.; Hagemann, H.; Battaglia, C. A stable 3 V all-solid-state sodium–ion battery based on a closo-borate electrolyte. Energy Environ. Sci. 2017, 10, 2609–2615. [Google Scholar] [CrossRef]
- Udovic, T.J.; Matsuo, M.; Tang, W.S.; Wu, H.; Stavila, V.; Soloninin, A.V.; Skoryunov, R.V.; Babanova, O.A.; Skripov, A.V.; Rush, J.J.; et al. Exceptional superionic conductivity in disordered sodium decahydro-closo-decaborate. Adv. Mater. 2014, 26, 7622–7626. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Liang, X.; Xia, Y.; Peng, L.; Jia, H.; Li, H.; Bai, L.; Feng, J.; Jiang, H.; et al. Rotational Cluster Anion Enabling Superionic Conductivity in Sodium-Rich Antiperovskite Na3OBH4. J. Am. Chem. Soc. 2019, 141, 5640–5644. [Google Scholar] [CrossRef]
- Dimitrievska, M.; Shea, P.; Kweon, K.E.; Bercx, M.; Varley, J.B.; Tang, W.S.; Skripov, A.V.; Stavila, V.; Udovic, T.J.; Wood, B.C. Carbon Incorporation and Anion Dynamics as Synergistic Drivers for Ultrafast Diffusion in Superionic LiCB11H12 and NaCB11H12. Adv. Energy Mater. 2018, 8, 1703422. [Google Scholar] [CrossRef]
- Tang, W.S.; Matsuo, M.; Wu, H.; Stavila, V.; Unemoto, A.; Orimo, S.-I.; Udovic, T.J. Stabilizing lithium and sodium fast-ion conduction in solid polyhedral-borate salts at device-relevant temperatures. Energy Storage Mater. 2016, 4, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Feuillade, G.; Perche, P. Ion-conductive macromolecular gels and membranes for solid lithium cells. J. Appl. Electrochem. 1975, 5, 63–69. [Google Scholar] [CrossRef]
- Chen, R.; Qu, W.; Guo, X.; Li, L.; Wu, F. The pursuit of solid-state electrolytes for lithium batteries: From comprehensive insight to emerging horizons. Mater. Horiz. 2016, 3, 487–516. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Ratner, M.A.; Johansson, P.; Shriver, D.F. Polymer Electrolytes: Ionic Transport Mechanisms and Relaxation Coupling. MRS Bull. 2000, 3, 31–37. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Hou, H.D.; Xu, Q.K.; Pang, Y.K.; Li, L.; Wang, J.L.; Zhang, C.; Sun, C.W. Efficient Storing Energy Harvested by Triboelectric Nanogenerators Using a Safe and Durable All-solid-state Sodium-ion Battery. Adv. Sci. 2017, 4, 1700072. [Google Scholar] [CrossRef]
- Hu, X.; Li, Z.; Zhao, Y.; Sun, J.; Zhao, Q.; Wang, J.; Tao, Z.; Chen, J. Quasi-solid state rechargeable Na-CO2 batteries with reduced graphene oxide Na anodes. Sci. Adv. 2017, 3, e1602396. [Google Scholar] [CrossRef] [Green Version]
- Nagasubramanian, G.; Stefano, S.D. 12-Crown-4 Ether-Assisted Enhancement of Ionic Conductivity and Interfacial Kinetics in Polyethylene Oxide Electrolytes. J. Electrochem. Soc. 1990, 12, 3830. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Wan, C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Source 1999, 2, 183–197. [Google Scholar] [CrossRef]
- Yu, Q.; Lu, Q.; Qi, X.; Zhao, S.; He, Y.-B.; Liu, L.; Li, J.; Zhou, D.; Hu, Y.-S.; Yang, Q.-H.; et al. Liquid electrolyte immobilized in compact polymer matrix for stable sodium metal anodes. Energy Storage Mater. 2019, 23, 610–616. [Google Scholar] [CrossRef]
- Zhou, D.; He, Y.-B.; Cai, Q.; Qin, X.; Li, B.; Du, H.; Yang, Q.-H.; Kang, F. Investigation of cyano resin-based gel polymer electrolyte: In situ gelation mechanism and electrode-electrolyte interfacial fabrication in lithium-ion battery. J. Mater. Chem. A 2014, 2, 20059–20066. [Google Scholar] [CrossRef]
- Zhang, W.; Yi, Q.; Li, S.; Sun, C. An ion-conductive Li7La3Zr2O12-based composite membrane for dendrite-free lithium metal batteries. J. Power Source 2020, 450, 227710. [Google Scholar] [CrossRef]
- Skaarup, S.; West, K.; Zachau-Christiansen, B. Mixed phase solid electrolytes. Solid State Ion. 1988, 28–30, 975–978. [Google Scholar] [CrossRef]
- Wieczorek, W. Modifications of crystalline structure of peo polymer electrolytes with ceramic additives. Solid State Ion. 1989, 3–4, 255–257. [Google Scholar] [CrossRef]
- Liu, L.; Qi, X.; Yin, S.; Zhang, Q.; Liu, X.; Suo, L.; Li, H.; Chen, L.; Hu, Y.-S. In Situ Formation of a Stable Interface in Solid-State Batteries. ACS Energy Lett. 2019, 4, 1650–1657. [Google Scholar] [CrossRef]
- Ni’mah, Y.L.; Cheng, M.-Y.; Cheng, J.H.; Rick, J.; Hwang, B.-J. Solid-state polymer nanocomposite electrolyte of TiO2/PEO/NaClO4 for sodium ion batteries. J. Power Source 2015, 278, 375–381. [Google Scholar] [CrossRef]
- Thakur, A.K.; Upadhyaya, H.M.; Hashmi, S.A. Polyethylene oxide based sodium ion conducting composite polymer electrolytes dispersed with Na2SiO3. Indian J. Pure Appl. Phys. 1999, 37, 302–305. [Google Scholar]
- Zhang, Z.; Zhang, Q.; Ren, C.; Luo, F.; Ma, Q.; Hu, Y.-S.; Zhou, Z.; Li, H.; Huang, X.; Chen, L. A ceramic/polymer composite solid electrolyte for sodium batteries. J. Mater. Chem. A 2016, 4, 15823–15828. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Su, X.; Lu, Y.X.; Hu, Y.S. A Composite Solid-state Polymer Electrolyte for Solid-state Sodium Batteries. J. Chin. Ceram. Soc. 2020, 48, 939–946. [Google Scholar]
- Colò, F.; Bella, F.; Nair, J.R.; Destro, M.; Gerbaldi, C. Cellulose-based novel hybrid polymer electrolytes for green and efficient Na-ion batteries. Electrochim. Acta 2015, 174, 185–190. [Google Scholar] [CrossRef]
- Gao, H.; Guo, B.; Song, J.; Park, K.; Goodenough, J.B. A Composite Gel-Polymer/Glass-Fiber Electrolyte for Sodium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1402235. [Google Scholar] [CrossRef]
- Villaluenga, I.; Bogle, X.; Greenbaum, S.; Gil de Muro, I.; Rojo, T.; Armand, M. Cation only conduction in new polymer-SiO2 nanohybrids: Na+ electrolytes. J. Mater. Chem. A 2013, 1, 8348–8352. [Google Scholar] [CrossRef]
- Lu, Y.; Alonso, J.A.; Yi, Q.; Lu, L.; Wang, Z.L.; Sun, C.W. A High-performance Monolithic Solid-State Sodium Battery with Ca2+ Doped Na3Zr2Si2PO12 Electrolyte. Adv. Energy Mater. 2019, 9, 1901205. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, C.W. Recent Advances in Dendrite-free Lithium Metal Anode for High-Performance Batteries. Phys. Chem. Chem. Phys. 2022, 24, 19996–20011. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.P.; Li, N.W.; Sun, C.W. High-performance sodium metal batteries with sodium-bismuth alloy anode. ACS Appl. Energy Mater. 2020, 3, 12607–12612. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, L.; Qiu, G.R.; Sun, C.W. Flexible Quasi-Solid-State Sodium Battery for Storing Pulse Electricity Harvested by Triboelectric Nanogenerators. ACS Appl. Mater. Interfaces 2020, 12, 39342–39351. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Xin, S.; Goodenough, J.B. Rechargeable Sodium All-Solid-State Battery. ACS Cent. Sci. 2017, 3, 52–57. [Google Scholar] [CrossRef]
- Yi, Q.; Lu, Y.; Sun, X.R.; Zhang, H.; Yu, H.L.; Sun, C.W. Fluorinated ether based electrolyte with low flammability enabling sodium-metal batteries with exceptional cycling stability. ACS Appl. Mater. Interfaces 2019, 11, 46965–46972. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, M.; Kang, P.; Zhu, T.; Yuan, H.; Lan, J.; Yang, X.; Sui, G. Self-Enhancing Gel Polymer Electrolyte by In Situ Construction for Enabling Safe Lithium Metal Battery. Adv. Sci. 2022, 9, e2103663. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.N.; Ma, Q.Y.; Sun, S.Y.; Yang, K.; Cai, Q.; Olsson, E.; Chen, X.; Wang, Z.; Abdelkader, A.M.; Li, Y.S.; et al. Highly aligned lithiophilic electrospun nanofiber membrane for the multiscale suppression of Li dendrite growth. eScience 2022, 2, 655–665. [Google Scholar] [CrossRef]
- Yi, Q.; Zhang, W.Q.; Li, S.Q.; Sun, C.W. A durable sodium battery with a flexible Na3Zr2Si2PO12-PVDF-HFP composite electrolyte and sodium/carbon cloth anode. ACS Appl. Mater. Interfaces 2018, 10, 35039–35046. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Stalin, S.; Zhao, C.Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Feng, L.L.; Yu, T.S.; Cheng, D.H.; Sun, C.W. Progress of Sodium Battery Failure Research. Sci. Sin. Chim. 2020, 50, 1801–1815. [Google Scholar]
- Eng, S.Y.S.; Soni, C.B.; Lum, Y.; Khoo, E.; Yao, Z.P.; Vineeth, S.K.; Kumar, V.; Lu, J.; Johnson, C.S.; Wolverton, C.; et al. Theory-guided experimental design in battery materials research. Sci. Adv. 2022, 8, eabm2422. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, Y.; Sun, H.; Yang, T.; Sun, Y.; Du, C.; Jia, P.; Ye, H.; Chen, J.; Peng, Q.; et al. In Situ Electrochemical Study of Na-O2/CO2 Batteries in an Environmental Transmission Electron Microscope. ACS Nano 2020, 14, 13232–13245. [Google Scholar] [CrossRef]
- Yang, M.; Bi, R.; Wang, J.; Yu, R.; Wang, D. Decoding lithium batteries through advanced in situ characterization techniques. Int. J. Miner. Metall. Mater. 2022, 29, 965–989. [Google Scholar] [CrossRef]
- Dixit, M.B.; Park, J.-S.; Kenesei, P.; Almer, J.; Hatzell, K.B. Status and prospect of in situ and operando characterization of solid-state batteries. Energy Environ. Sci. 2021, 14, 4672–4711. [Google Scholar] [CrossRef]
- Hou, D.; Xia, D.; Gabriel, E.; Russell, J.A.; Graff, K.; Ren, Y.; Sun, C.-J.; Lin, F.; Liu, Y.; Xiong, H. Spatial and Temporal Analysis of Sodium-Ion Batteries. ACS Energy Letters 2021, 6, 4023–4054. [Google Scholar] [CrossRef]
- Liu, D.; Shadike, Z.; Lin, R.; Qian, K.; Li, H.; Li, K.; Wang, S.; Yu, Q.; Liu, M.; Ganapathy, S.; et al. Review of Recent Development of In Situ/Operando Characterization Techniques for Lithium Battery Research. Adv. Mater. 2019, 31, e1806620. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Tian, X.D.; Lin, J.S.; Dong, J.C.; Lin, X.M.; Li, J.F. In situ Raman, FTIR, and XRD spectroscopic studies in fuel cells and rechargeable batteries. Nano Res. 2022. [Google Scholar] [CrossRef]
- Bak, S.-M.; Shadike, Z.; Lin, R.; Yu, X.; Yang, X.-Q. In situ/operando synchrotron-based X-ray techniques for lithium-ion battery research. NPG Asia Mater. 2018, 10, 563–580. [Google Scholar] [CrossRef] [Green Version]
- Bragg, W. The structure of some crystals as indicated by their diffraction of X-rays. Proc. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1997, 89, 248–277. [Google Scholar]
- Ameh, E.S. A review of basic crystallography and x-ray diffraction applications. Int. J. Adv. Manuf. Technol. 2019, 105, 3289–3302. [Google Scholar] [CrossRef]
- Xia, M.; Liu, T.; Peng, N.; Zheng, R.; Cheng, X.; Zhu, H.; Yu, H.; Shui, M.; Shu, J. Lab-Scale In Situ X-ray Diffraction Technique for Different Battery Systems: Designs, Applications, and Perspectives. Small Methods 2019, 3, 1900119. [Google Scholar] [CrossRef]
- Yu, W.; Yang, W.; Liu, R.; Qin, L.; Lei, Y.; Liu, L.; Zhai, D.; Li, B.; Kang, F. A soluble phenolic mediator contributing to enhanced discharge capacity and low charge overpotential for lithium-oxygen batteries. Electrochem. Commun. 2017, 79, 68–72. [Google Scholar] [CrossRef]
- Sheng, C.; Yu, F.; Wu, Y.; Peng, Z.; Chen, Y. Disproportionation of Sodium Superoxide in Metal-air Batteries. Angew. Chem. Int. Ed. Engl. 2018, 57, 9906–9910. [Google Scholar] [CrossRef]
- Ganapathy, S.; Adams, B.D.; Stenou, G.; Anastasaki, M.S.; Goubitz, K.; Miao, X.F.; Nazar, L.F.; Wagemaker, M. Nature of Li2O2 oxidation in a Li-O2 battery revealed by operando X-ray diffraction. J. Am. Chem. Soc. 2014, 136, 16335–16344. [Google Scholar] [CrossRef]
- Lin, F.; Liu, Y.; Yu, X.; Cheng, L.; Singer, A.; Shpyrko, O.G.; Xin, H.L.; Tamura, N.; Tian, C.; Weng, T.C.; et al. Synchrotron X-ray Analytical Techniques for Studying Materials Electrochemistry in Rechargeable Batteries. Chem. Rev. 2017, 117, 13123–13186. [Google Scholar] [CrossRef]
- Sun, C.W.; López, C.A.; Alonso, J.A. Elucidating the Diffusion Pathway of Protons in Ammonium Polyphosphate: A potential Electrolyte for Intermediate Temperature Fuel Cells. J. Mater. Chem. A 2017, 5, 7839–7844. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.W.; Chen, L.L.; Shi, S.Q.; Reeb, B.; López, C.A.; Alonso, J.A.; Stimming, U. Visualization of the Diffusion Pathway of Protons in the Ammonium Polyphosphate for Intermediate Temperature Fuel Cells. Inorg. Chem. 2018, 57, 676–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.Q.; Meng, X.Y.; Alonso, J.A.; Fernández-Díaz, M.T.; Sun, C.W.; Wang, Z.L. Structural and Electrochemical Properties of LiMn0.6Fe0.4PO4 as a Cathode Material for Flexible Lithium-ion Batteries and Self-charging Power Pack. Nano Energy 2018, 52, 510–516. [Google Scholar] [CrossRef]
- Lu, Y.; López, C.A.; Wang, J.; Alonso, J.A.; Sun, C.W. Insight into the Structure and Functional Application of Mg doped Na0.5Bi0.5TiO3 electrolyte for Solid Oxide Fuel Cells. J. Alloys Compd. 2018, 752, 213–219. [Google Scholar] [CrossRef]
- Gong, Y.D.; Sun, C.W.; Huang, Q.A.; Alonso, J.A.; Fernández-Díaz, M.T.; Chen, L.Q. Dynamic octahedral breathing in oxygen-deficient Ba0.9Co0.7Fe0.2Nb0.1O3−δ perovskite performing as a cathode for intermediate-temperature solid oxide fuel cells. Inorg. Chem. 2016, 55, 3091–3097. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, H.R.; Sun, C.W.; Liu, L.L.; Alonso, J.A.; Fernández-Díaz, M.T.; Chen, L.Q. Insight into the structure and functional application of Sr0.95Ce0.05CoO3−δ cathode for solid oxide fuel cells. Inorg. Chem. 2015, 54, 3477–3485. [Google Scholar] [CrossRef]
- Ma, Z.H.; Wang, Y.S.; Sun, C.W.; Alonso, J.A.; Fernández-Díaz, M.T.; Chen, L.Q. Experimental visualization of the diffusion pathway of sodium ions in the Na3[Ti2P2O10F] anode for sodium-ion battery. Sci. Rep. 2014, 4, 7231. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, C.W.; Gong, Y.D.; Zhang, H.R.; Alonso, J.A.; Fernández-Díaz, M.T.; Wang, Z.L.; Goodenough, J.B. Imaging of the diffusion pathway of Al3+ ion in NASICON-type (Al0.2Zr0.8)20/19Nb(PO4)3 electrolyte for high-temperature solid-state Al batteries. Chin. Phys. B 2018, 27, 128201. [Google Scholar] [CrossRef]
- Rong, X.; Liu, J.; Hu, E.; Liu, Y.; Wang, Y.; Wu, J.; Yu, X.; Page, K.; Hu, Y.-S.; Yang, W.; et al. Structure-Induced Reversible Anionic Redox Activity in Na Layered Oxide Cathode. Joule 2018, 2, 125–140. [Google Scholar] [CrossRef]
- Castellanos, M.M.; McAuley, A.; Curtis, J.E. Investigating Structure and Dynamics of Proteins in Amorphous Phases Using Neutron Scattering. Comput. Struct. Biotechnol. J. 2017, 15, 117–130. [Google Scholar] [CrossRef]
- Matthey, J. In the Lab: Synchrotron Radiation Based Solid Phase Characterisation of Industrial Catalysts and Materials. Johnson Matthey Technol. Rev. 2016, 60, 158–160. [Google Scholar]
- Wang, X.; Tan, S.; Yang, X.-Q.; Hu, E. Pair distribution function analysis: Fundamentals and application to battery materials. Chin. Phys. B 2020, 29, 028802. [Google Scholar] [CrossRef]
- Castillo-Blas, C.; Moreno, J.M.; Romero-Muniz, I.; Platero-Prats, A.E. Applications of pair distribution function analyses to the emerging field of non-ideal Metal-organic framework materials. Nanoscale 2020, 12, 15577–15587. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Folastre, N.; Bolomont, M.; Jamali, A.; Morcrette, M.; Davoisne, C. Understanding the Battery Degradation Mechanism in All-solid-state Batteries via In-situ SEM. Microsc. Microanal. 2021, 27, 105–106. [Google Scholar] [CrossRef]
- Han, S.; Cai, C.; Yang, F.; Zhu, Y.; Sun, Q.; Zhu, Y.G.; Li, H.; Wang, H.; Shao-Horn, Y.; Sun, X.; et al. Interrogation of the Reaction Mechanism in a Na-O2 Battery Using In Situ Transmission Electron Microscopy. ACS Nano 2020, 14, 3669–3677. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Y.; Liu, Q.; Yang, T.; Du, C.; Jia, P.; Wang, Z.; Tang, Y.; Li, Y.; Shen, T.; et al. Probing the charging and discharging behavior of K-CO2 nanobatteries in an aberration corrected environmental transmission electron microscope. Nano Energy 2018, 53, 544–549. [Google Scholar] [CrossRef]
- Liang, Z.; Zou, Q.; Wang, Y.; Lu, Y.C. Recent Progress in Applying In Situ/Operando Characterization Techniques to Probe the Solid/Liquid/Gas Interfaces of Li-O2 Batteries. Small Methods 2017, 1, 1700150. [Google Scholar] [CrossRef]
- Cortes, H.A.; Corti, H.R. In-situ characterization of discharge products of lithium-oxygen battery using Flow Electrochemical Atomic Force Microscopy. Ultramicroscopy 2021, 230, 113369. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Xie, J.; Wang, C.H.; Yang, Y.W.; Lu, Y.-C. Electrochemical reduction of CO2 in ionic liquid: Mechanistic study of Li–CO2 batteries via in situ ambient pressure X-ray photoelectron spectroscopy. Nano Energy 2021, 83, 105830. [Google Scholar] [CrossRef]
- Wang, M.; Árnadóttir, L.; Xu, Z.J.; Feng, Z. In Situ X-ray Absorption Spectroscopy Studies of Nanoscale Electrocatalysts. Nano-Micro Lett. 2019, 11, 47. [Google Scholar] [CrossRef]
- Kimura, Y.; Fakkao, M.; Nakamura, T.; Okumura, T.; Ishiguro, N.; Sekizawa, O.; Nitta, K.; Uruga, T.; Tada, M.; Uchimoto, Y.; et al. Influence of Active Material Loading on Electrochemical Reactions in Composite Solid-State Battery Electrodes Revealed by Operando 3D CT-XANES Imaging. ACS Appl. Energy Mater. 2020, 3, 7782–7793. [Google Scholar] [CrossRef]
- Gao, R.; Zhou, D.; Ning, D.; Zhang, W.; Huang, L.; Sun, F.; Schuck, G.; Schumacher, G.; Hu, Z.; Liu, X. Probing the Self-Boosting Catalysis of LiCoO2 in Li-O2 Battery with Multiple In Situ/Operando Techniques. Adv. Funct. Mater. 2020, 30, 2002223. [Google Scholar] [CrossRef]
- Ding, S.; Yu, X.; Ma, Z.-F.; Yuan, X. A review of rechargeable aprotic lithium–oxygen batteries based on theoretical and computational investigations. J. Mater. Chem. A 2021, 9, 8160–8194. [Google Scholar] [CrossRef]
- Deng, Q.; Yang, Y.; Qu, S.; Wang, W.; Zhang, Y.; Ma, X.; Yan, W.; Zhang, Y. Electron structure and reaction pathway regulation on porous cobalt-doped CeO2/graphene aerogel: A free-standing cathode for flexible and advanced Li-CO2 batteries. Energy Storage Mater. 2021, 42, 484–492. [Google Scholar] [CrossRef]
- Jiang, H.R.; Tan, P.; Liu, M.; Zeng, Y.K.; Zhao, T.S. Unraveling the Positive Roles of Point Defects on Carbon Surfaces in Nonaqueous Lithium-Oxygen Batteries. J. Phys. Chem. C 2016, 120, 18394–18402. [Google Scholar] [CrossRef]
- Yuan, M.W.; Sun, Z.M.; Yang, H.; Wang, D.; Liu, Q.M.; Nan, C.Y.; Li, H.F.; Sun, G.B.; Chen, S.W. Self-Catalyzed Rechargeable Lithium-Air Battery by in-situ Metal Ion Doping of Discharge Products: A Combined Theoretical and Experimental Study. Energy Environ. Mater. 2021, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.K.; Li, J.; Shen, Q.Y.; Zhang, J.; He, P.G.; Qu, X.H.; Liu, Y.C. Advanced characterizations and measurements for sodium-ion batteries with NASICON-type cathode materials. eScience 2022, 2, 10–31. [Google Scholar] [CrossRef]
- Jain, A.; Hautier, G.; Moore, C.J.; Ping Ong, S.; Fischer, C.C.; Mueller, T.; Persson, K.A.; Ceder, G. A high-throughput infrastructure for density functional theory calculations. Comp. Mater. Sci. 2011, 50, 2295–2310. [Google Scholar] [CrossRef]
- Wang, H.S.; Ji, Y.J.; Li, Y.Y. Simulation and design of energy materials accelerated by machine learning. WIREs Comput. Mol. Sci. 2019, 10, e1421. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Sun, C.; Lu, L.; Jiao, L. Recent Progress and Perspectives of Solid State Na-CO2 Batteries. Batteries 2023, 9, 36. https://doi.org/10.3390/batteries9010036
Wang Z, Sun C, Lu L, Jiao L. Recent Progress and Perspectives of Solid State Na-CO2 Batteries. Batteries. 2023; 9(1):36. https://doi.org/10.3390/batteries9010036
Chicago/Turabian StyleWang, Zelin, Chunwen Sun, Liang Lu, and Lifang Jiao. 2023. "Recent Progress and Perspectives of Solid State Na-CO2 Batteries" Batteries 9, no. 1: 36. https://doi.org/10.3390/batteries9010036
APA StyleWang, Z., Sun, C., Lu, L., & Jiao, L. (2023). Recent Progress and Perspectives of Solid State Na-CO2 Batteries. Batteries, 9(1), 36. https://doi.org/10.3390/batteries9010036