Three-Dimensional Nanoporous CNT@Mn3O4 Hybrid Anode: High Pseudocapacitive Contribution and Superior Lithium Storage
Abstract
:1. Introduction
2. Experimental Details
2.1. Synthesis of CNT@Mn3O4 Hybrid Electrode
2.2. Material Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Microstructural Studies
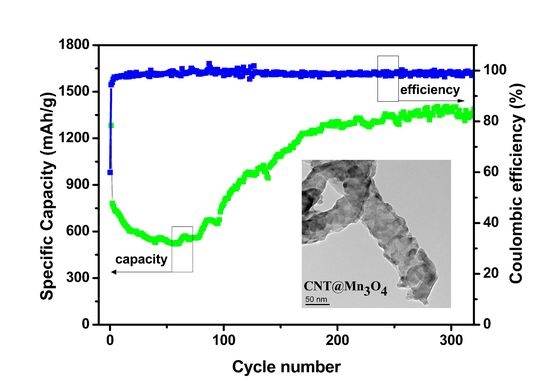
3.2. Electrochemical Studies
- Mn3O4 + Li+ + e− → LiMn3O4 (1.5–0.5V vs. Li/Li+);
- Mn3O4 + Li+ + e− → Li2O + 3MnO (1.5–0.5V vs. Li/Li+);
- MnO + 2Li+ + 2e− → Li2O + Mn (0.5–0.0 V vs. Li/Li+).
- Li2O + Mn → MnO + 2Li+ + 2e− (0.5–3.0 V vs. Li/Li+).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, S.; Dong, F.; Yue, P.; Han, P.; Yang, J. Research Progress on Nanostructured Metal Oxides as Anode Materials for Li-ion Battery. J. Inorg. Mater. 2020, 35, 134. [Google Scholar]
- Marques, O.J.B.J.; Walter, M.D.; Timofeeva, E.V.; Segre, C.U. Effect of Initial Structure on Performance of High-Entropy Oxide Anodes for Li-Ion Batteries. Batteries 2023, 9, 115. [Google Scholar] [CrossRef]
- Cao, K.; Jin, T.; Yang, L.; Jiao, L. Recent progress in conversion reaction metal oxide anodes for Li-ion batteries. Mater. Chem. Front. 2017, 1, 2213–2242. [Google Scholar] [CrossRef]
- Rahman, M.M.; Marwani, H.M.; Algethami, F.K.; Asiri, A.M. Comparative performance of hydrazine sensors developed with Mn3O4/carbon-nanotubes, Mn3O4/graphene-oxides and Mn3O4/carbon-black nanocomposites. Mater. Express 2017, 7, 169–179. [Google Scholar] [CrossRef]
- Xu, L.; Chen, X.; Zeng, L.; Liu, R.; Zheng, C.; Qian, Q.; Chen, Q. Synthesis of hierarchical Mn3O4 microsphere composed of ultrathin nanosheets and its excellent long-term cycling performance for lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2019, 30, 3055–3060. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, Z.; Hu, W.; Liu, C.; Wang, X.; Zhao, Z.; Ye, W. Microwave-assisted hydrothermal synthesis of Mn3O4/reduced graphene oxide composites for efficiently catalytic reduction of 4-nitrophenol in wastewater. J. Taiwan Inst. Chem. Eng. 2018, 84, 101–109. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Wang, S.; Wang, Z.; Wen, Z.; Ji, S.; Sun, J. An amorphous hierarchical MnO2/acetylene black composite with boosted rate performance as an anode for lithium-ion batteries. Dalton Trans. 2021, 50, 10749–10757. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Zhao, D.L.; Cheng, X.W.; Ding, Z.W.; Hu, T.; Meng, S. Nanorod Mn3O4 anchored on graphene nanosheet as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. J. Alloys Compd. 2017, 728, 383–390. [Google Scholar] [CrossRef]
- Wang, B.; Li, F.; Wang, X.; Wang, G.; Wang, H.; Bai, J. Mn3O4 nanotubes encapsulated by porous graphene sheets with enhanced electrochemical properties for lithium/sodium-ion batteries. Chem. Eng. J. 2019, 364, 57–69. [Google Scholar] [CrossRef]
- Varghese, S.P.; Babu, B.; Prasannachandran, R.; Antony, R.; Shaijumon, M.M. Enhanced electrochemical properties of Mn3O4/graphene nanocomposite as efficient anode material for lithium ion batteries. J. Alloys Compd. 2019, 780, 588–596. [Google Scholar] [CrossRef]
- Peng, H.-J.; Hao, G.-X.; Chu, Z.-H.; Lin, J.; Lin, X.-M.; Cai, Y.-P. Mesoporous Mn3O4/C Microspheres Fabricated from MOF Template as Advanced Lithium-Ion Battery Anode. Cryst. Growth Des. 2017, 17, 5881–5886. [Google Scholar] [CrossRef]
- Gangaraju, D.; Sridhar, V.; Lee, I.; Park, H. Graphene—Carbon nanotube—Mn3O4 mesoporous nano-alloys as high capacity anodes for lithium-ion batteries. J. Alloys Compd. 2017, 699, 106–111. [Google Scholar] [CrossRef]
- Deng, Y.; Wan, L.; Xie, Y.; Qin, X.; Chen, G. Recent advances in Mn-based oxides as anode materials for lithium ion batteries. RSC Adv. 2014, 4, 23914–23935. [Google Scholar] [CrossRef]
- Fang, H.; Zou, W.; Yan, J.; Xing, Y.; Zhang, S. Facile Fabrication of Fe2O3 Nanoparticles Anchored on Carbon Nanotubes as High-Performance Anode for Lithium-Ion Batteries. ChemElectroChem 2018, 5, 2458–2463. [Google Scholar] [CrossRef]
- Kuila, B.K.; Zaeem, S.M.; Daripa, S.; Kaushik, K.; Gupta, S.K.; Das, S. Mesoporous Mn3O4 coated reduced graphene oxide for high-performance supercapacitor applications. Mater. Res. Express 2018, 6, 015037–015046. [Google Scholar] [CrossRef]
- Rosaiah, P.; Zhu, J.; Shaik, D.P.; Hussain, O.M.; Qiu, Y.; Zhao, L. Reduced graphene oxide/Mn3O4 nanocomposite electrodes with enhanced electrochemical performance for energy storage applications. J. Electroanal. Chem. 2017, 794, 78–85. [Google Scholar]
- Zhou, Y.; Guo, L.; Shi, W.; Zou, X.; Xiang, B.; Xing, S. Rapid Production of Mn3O4/rGO as an Efficient Electrode Material for Supercapacitor by Flame Plasma. Materials 2018, 11, 881. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yue, W.; Li, W.; Zhao, J.; Zhang, Y.; Gao, Y.; Gao, N.; Feng, D.; Wu, B.; Wang, B. Rational design of 3D net-like carbon based Mn3O4 anode materials with enhanced lithium storage performance. New J. Chem. 2022, 46, 13220–13227. [Google Scholar] [CrossRef]
- Cao, K. Mn3O4 nanoparticles anchored on carbon nanotubes as anode material with enhanced lithium storage. J. Alloys Compd. Interdiscip. J. Mater. Sci. Solid-State Chem. Phys. 2021, 854, 157176–157179. [Google Scholar] [CrossRef]
- Seong, C.-Y.; Park, S.-K.; Bae, Y.; Yoo, S.; Piao, Y. An acid-treated reduced graphene oxide/Mn3O4 nanorod nanocomposite as an enhanced anode material for lithium ion batteries. RSC Adv. 2017, 7, 37502–37507. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Yue, J.L.; Guo, Q.; Xia, Q.; Hui, X. Highly Porous Mn3O4 Micro/Nanocuboids with In Situ Coated Carbon as Advanced Anode Material for Lithium-Ion Batteries. Small 2018, 14, 1704296. [Google Scholar]
- Shah, H.U.; Wang, F.; Javed, M.S.; Shaheen, N.; Saleem, M.; Li, Y. Hydrothermal synthesis of reduced graphene oxide-Mn3O4 nanocomposite as an efficient electrode materials for supercapacitors. Ceram. Int. 2017, 44, 3580–3584. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Gim, J.; Kim, S.; Song, J.; Duong, P.T.; Jo, J.; Baboo, J.P.; Xiu, Z.; Mathew, V.; Kim, J. One-Step Pyro-Synthesis of a Nanostructured Mn3O4/C Electrode with Long Cycle Stability for Rechargeable Lithium-Ion Batteries. Chemistry 2016, 22, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Hou, H.; Yang, Y.; Zhang, Y.; Yang, X.; Chen, Q.; Ji, X. Electrochemically Alternating Voltage Induced Mn3O4/Graphite Powder Composite with Enhanced Electrochemical Performances for Lithium-ion Batteries. Electrochim. Acta 2015, 155, 157–163. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, D.; Zhao, R.; Gao, A.; Chen, H. Synthesis of a novel structured Mn3O4@C composite and its performance as anode for lithium ion battery. Mater. Lett. 2017, 198, 97–100. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Y.; Li, Q.; Wang, D.; Zhang, F.; Yang, Y.; Yu, Y. SnO2@C@VO2 Composite Hollow Nanospheres as an Anode Material for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 14993–14998. [Google Scholar] [CrossRef]
- Thauer, E.; Shi, X.; Zhang, S.; Chen, X.; Deeg, L.; Wenelska, K.; Mijowska, E.; Lund, H.; Kaiser, M.J. Mn3O4 encapsulated in hollow carbon spheres coated by graphene layer for enhanced magnetization and lithium-ion batteries performance. Energy 2021, 217, 119399–119420. [Google Scholar] [CrossRef]
- Oloore, L.E.; Gondal, M.A.; Popoola, A.; Popoola, I.K. Pseudocapacitive contributions to enhanced electrochemical energy storage in hybrid perovskite-nickel oxide nanoparticles composites electrodes. Electrochim. Acta 2020, 361, 137082–137091. [Google Scholar] [CrossRef]
- Peng, L.; Hao, Q.; Xia, X.; Wu, L.; Xin, W. Hollow Amorphous MnSnO3 Nanohybrid with Nitrogen-Doped Graphene for High-Performance Lithium Storage. Electrochim. Acta 2016, 214, 1–10. [Google Scholar]
- Wang, Y.; Rao, S.; Mao, P.; Zhang, F.; Xiao, P.; Peng, L.; Zhu, Q. Controlled synthesis of Fe3O4@C@manganese oxides (MnO2, Mn3O4 and MnO) hierarchical hollow nanospheres and their superior lithium storage properties. Electrochim. Acta 2020, 337, 135739–135777. [Google Scholar] [CrossRef]
- Yan, D.-J.; Zhu, X.-D.; Mao, Y.-C.; Qiu, S.-Y.; Gu, L.-L.; Feng, Y.-J.; Sun, K.-N. Hierarchically organized CNT@TiO2@Mn3O4 nanostructures for enhanced lithium storage performance. J. Mater. Chem. A 2017, 5, 17048–17055. [Google Scholar] [CrossRef]
- Gao, D.D.; Luo, S.S.; Zhang, Y.H.; Liu, J.Y.; Wu, H.M.; Wang, S.Q.; He, P.X. Mn3O4/carbon nanotubes nanocomposites as improved anode materials for lithium-ion batteries. J. Solid State Electrochem. 2018, 22, 3409–3417. [Google Scholar] [CrossRef]
- Liu, P.; Xia, X.; Lei, W.; Hao, Q. Rational synthesis of highly uniform hollow core–shell Mn3O4/CuO@TiO2 submicroboxes for enhanced lithium storage performance. Chem. Eng. J. 2017, 316, 214–224. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Deng, X.; He, C.; Liu, E.; He, F.; Shi, C.; Zhao, N. Ultrathin-Nanosheet-Induced Synthesis of 3D Transition Metal Oxides Networks for Lithium Ion Battery Anodes. Adv. Funct. Mater. 2017, 27, 1605017. [Google Scholar] [CrossRef]
- Sun, X.; Si, W.; Liu, X.; Deng, J.; Schmidt, O.G. Multifunctional ni/nio hybrid nanomembranes as anode materials for high-rate li-ion batteries. Nano Energy 2014, 9, 168–175. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, C.; Li, Y. One-step microwave preparation of a Mn3O4 nanoparticles/exfoliated graphite composite as superior anode materials for Li-ion batteries. Chem. Phys. Lett. 2017, 673, 19–23. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Yuan, L.-X.; Shao, Q.-G.; Huang, F.; Huang, Y.-H. Mn3O4 nanocrystals anchored on multi-walled carbon nanotubes as high-performance anode materials for lithium-ion batteries. Mater. Lett. 2012, 80, 110–113. [Google Scholar] [CrossRef]
- Gan, Z.; Yin, J.; Xu, X.; Cheng, Y.; Yu, T. Nanostructure and Advanced Energy Storage: Elaborate Material Designs Lead to High-Rate Pseudocapacitive Ion Storage. ACS Nano 2022, 16, 5131–5152. [Google Scholar] [CrossRef]
- Xiong, P.; Ma, R.; Sakai, N.; Sasaki, T. Genuine Unilamellar Metal Oxide Nanosheets Confined in a Superlattice-like Structure for Superior Energy Storage. ACS Nano 2018, 12, 1768–1777. [Google Scholar] [CrossRef]
- Yoon, E.S.; Choi, B.G.; Jeon, H.J. Highly ordered nanoscale phosphomolybdate-grafted polyaniline/metal hybrid layered structures prepared via secondary sputtering phenomenon as high-performance pseudocapacitor electrodes. Phys. Scr. 2021, 96, 125882. [Google Scholar] [CrossRef]
- Meng, X.; Guan, Z.; Zhao, J.; Cai, Z.; Li, S.; Bian, L.; Song, Y.; Guo, D.; Liu, X. Lithium-pre-intercalated T-Nb2O5/graphene composite promoting pseudocapacitive performance for ultralong lifespan capacitors. Chem. Eng. J. 2022, 43, 438–446. [Google Scholar] [CrossRef]
- Chu, Y.; Guo, L.; Xi, B.; Feng, Z.; Wu, F.; Lin, Y.; Liu, J.; Sun, D.; Feng, J.; Qian, Y.; et al. Embedding MnO@Mn3O4 Nanoparticles in an N-Doped-Carbon Framework Derived from Mn-Organic Clusters for Efficient Lithium Storage. Adv. Mater. 2018, 30, 1704244. [Google Scholar] [CrossRef] [PubMed]
- Avvaru, V.S.; Fernandez, I.J.; Feng, W.; Hinder, S.J.; Rodríguez, M.C.; Etacheri, V. Extremely pseudocapacitive interface engineered CoO@3D-NRGO hybrid anodes for high energy/power density and ultralong life lithium-ion batteries. Carbon 2021, 171, 869–881. [Google Scholar] [CrossRef]
- Vincent, M.; Avvaru, V.S.; Rodriguez, M.C.; Haranczyk, M.; Etacheri, V. High-rate and ultralong-life Mg-Li hybrid batteries based on highly pseudocapacitive dual-phase TiO2 nanosheet cathodes. J. Power Sources 2021, 15, 506. [Google Scholar] [CrossRef]
- Wang, X. Facile synthesis of Mn3O4 hollow polyhedron wrapped by multiwalled carbon nanotubes as a high-efficiency microwave absorber. Ceram. Int. 2020, 46, 6550–6559. [Google Scholar] [CrossRef]
- Choudhury, B.J.; Roy, K.; Moholkar, V.S. Improvement of Supercapacitor Performance through Enhanced Interfacial Interactions Induced by Sonication. Ind. Eng. Chem. Res. 2021, 60, 7611–7623. [Google Scholar] [CrossRef]
- Bhagwan, J.; Sahoo, A.; Yadav, K.L.; Sharma, Y. Porous, one dimensional and High Aspect Ratio Mn3O4 Nanofibers: Fabrication and Optimization for Enhanced Supercapacitive Properties. Electrochim. Acta 2015, 174, 992–1001. [Google Scholar] [CrossRef]
- Wang, J.G.; Jin, D.; Zhou, R.; Li, X.; Liu, X.R.; Shen, C.; Xie, K.; Li, B.; Kang, F.; Wei, B. Highly flexible graphene/Mn3O4 nanocomposite membrane as advanced anodes for li-ion batteries. Acs Nano 2016, 10, 6227–6234. [Google Scholar] [CrossRef]
- Dong, S.; Chang, C.; Liang, Z.; Zhang, Z.; An, L. Electroless deposition of carbon nanotubes doped with nickel and their electrical contact properties. J. Xian Univ. 2020, 47, 88–94. [Google Scholar]
- Zhang, J.; Chu, R.X.; Chen, Y.L.; Zeng, Y.; Zhang, Y.; Guo, H. Porous carbon encapsulated Mn3O4 for stable lithium storage and its ex-situ XPS study. Electrochim. Acta 2019, 319, 518–526. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, W.; Fang, H.; Ma, T.; Zhao, Y.; Wang, L.; Jia, X.; Zhang, L. Three-Dimensional Nanoporous CNT@Mn3O4 Hybrid Anode: High Pseudocapacitive Contribution and Superior Lithium Storage. Batteries 2023, 9, 389. https://doi.org/10.3390/batteries9070389
Zou W, Fang H, Ma T, Zhao Y, Wang L, Jia X, Zhang L. Three-Dimensional Nanoporous CNT@Mn3O4 Hybrid Anode: High Pseudocapacitive Contribution and Superior Lithium Storage. Batteries. 2023; 9(7):389. https://doi.org/10.3390/batteries9070389
Chicago/Turabian StyleZou, Wei, Hua Fang, Tengbo Ma, Yanhui Zhao, Lixia Wang, Xiaodong Jia, and Linsen Zhang. 2023. "Three-Dimensional Nanoporous CNT@Mn3O4 Hybrid Anode: High Pseudocapacitive Contribution and Superior Lithium Storage" Batteries 9, no. 7: 389. https://doi.org/10.3390/batteries9070389
APA StyleZou, W., Fang, H., Ma, T., Zhao, Y., Wang, L., Jia, X., & Zhang, L. (2023). Three-Dimensional Nanoporous CNT@Mn3O4 Hybrid Anode: High Pseudocapacitive Contribution and Superior Lithium Storage. Batteries, 9(7), 389. https://doi.org/10.3390/batteries9070389