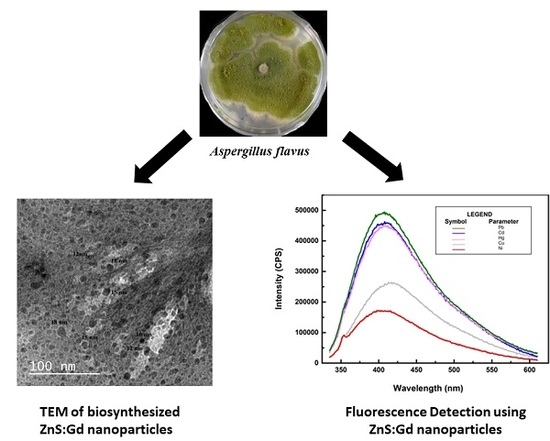
Bioinspired ZnS:Gd Nanoparticles Synthesized from an Endophytic Fungi Aspergillus flavus for Fluorescence-Based Metal Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Synthesis of Gd-Doped ZnS Nanoparticles
2.2. Detection of Metals by ZnS and ZnS:Gd Nanoparticles
3. Results and Discussion
3.1. Comparison of ZnS and ZnS:Gd Nanoparticles Based on EDS, Elemental Analysis, and AFM
3.2. Morphological and Optical Characterization
3.3. Sensing of Metals Using ZnS Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Onwudiwe, D.C.; Krüger, T.P.J.; Strydom, C.A. Laser assisted solid state reaction for the synthesis of ZnS and CdS nanoparticles from metal xanthate. Mater. Lett. 2014, 116, 154–159. [Google Scholar] [CrossRef]
- Prasanth, S.; Irshad, P.; Raj, D.R.; Vineeshkumar, T.V.; Philip, R.; Sudarsanakumar, C. Nonlinear optical property and fluorescence quenching behavior of PVP capped ZnS nanoparticles co-doped with Mn2+ and Sm3+. J. Lumin. 2015, 166, 167–175. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Baker, S.; Satish, S. Biosynthesis of gold nanoparticles by Pseudomonas veronii AS41G inhabiting Annona squamosa L. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Srinath, B.S.; Rai, R.V. Biosynthesis of gold nanoparticles using extracellular molecules produced by enterobacter aerogenes and their catalytic study. J. Clust. Sci. 2015, 26, 1483–1494. [Google Scholar] [CrossRef]
- Anand, K.V.; Chinnu, M.K.; Kumar, R.M.; Mohan, R.; Jayavel, R. Thermal stability and optical properties of HMTA capped zinc sulfide nanoparticles. J. Alloys Compd. 2010, 496, 665–668. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Park, H.H. Colorimetric detection of manganese (II) ions using gold/dopa nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 131, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Turdean, G.L. Design and development of biosensors for the detection of heavy metal toxicity. Int. J. Electrochem. 2011, 2011, 1–15. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Nigam, V.K.; Shukla, P. Enzyme based biosensors for detection of environmental pollutants—A review. J. Microbiol. Biotechnol. 2015, 25, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Petänen, T.; Romantschuk, M. Use of bioluminescent bacterial sensors as an alternative method for measuring heavy metals in soil extracts. Anal. Chim. Acta 2002, 456, 55–61. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors—Sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Yang, C.-Y.; Sun, R.-L.; Cheng, Y.-F.; Kao, W.-C.; Yang, P.-C. Rapid single cell detection of Staphylococcus aureus by aptamer-conjugated gold nanoparticles. Sci. Rep. 2013, 3, 1863. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, A.P. Perspectives on the physical chemistry of semiconductor nanocrystals. J. Phys. Chem. 1996, 100, 13226–13239. [Google Scholar] [CrossRef]
- Mary, J.; Mohan, R.; Bhat, U. Biosynthesis of lead selenide quantum rods in marine Aspergillus terreus. Mater. Lett. 2014, 124, 279–281. [Google Scholar] [CrossRef]
- Senapati, U.S.; Jha, D.K.; Sarkar, D. Green synthesis and characterization of ZnS nanoparticles. Res. J. Phys. Sci. Res. J. Phys. Sci. 2013, 1, 2320–4796. [Google Scholar]
- Advances, R.S.C.; Rourkela, T. Effect of silver doping on TiO2, CdS, and ZnS nanoparticles for the photocatalytic degradation of metronidazole under visible light. RSC Adv. Pap. 2014. [Google Scholar] [CrossRef]
- Shakir, M.; Faraz, M.; Sherwani, M.A.; Al-Resayes, S.I. Photocatalytic degradation of the Paracetamol drug using Lanthanum doped ZnO nanoparticles and their in-vitro cytotoxicity assay. J. Lumin. 2016, 176, 159–167. [Google Scholar] [CrossRef]
- Moon, J.-W.; Rawn, C.J.; Rondinone, A.J.; Love, L.J.; Roh, Y.; Everett, S.M.; Lauf, R.J.; Phelps, T.J. Large-scale production of magnetic nanoparticles using bacterial fermentation. J. Ind. Microbiol. Biotechnol. 2010, 37, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Poornaprakash, B.; Chalapathi, U.; Babu, S.; Park, S.H. Structural, morphological, optical, and magnetic properties of Gd-doped and (Gd, Mn) co-doped ZnO nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 93, 111–115. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Byon, C.; Jeon, S. Enhanced photocatalytic activity of ZnS nanoparticles loaded with MoS2 nanoflakes by self-assembly approach. Phys. B Condens. Matter 2016, 502, 103–112. [Google Scholar] [CrossRef]
- Uddandarao, P. ZnS semiconductor quantum dots production by an endophytic fungus Aspergillus flavus. Mater. Sci. Eng. B 2016, 207, 26–32. [Google Scholar] [CrossRef]
- Poornaprakash, B.; Chalapathi, U.; Reddeppa, M.; Park, S.H. Effect of Gd doping on the structural, luminescence and magnetic properties of ZnS nanoparticles synthesized by the hydrothermal method. Superlattices Microstruct. 2016, 97, 104–109. [Google Scholar] [CrossRef]
- Vogel, R.; Vogel, R.; Meredith, P.; Harvey, M.D.; Rubinsztein-dunlop, H. Absorption and fluorescence spectroscopy of rhodamine 6G in titanium dioxide nanocomposites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 245–249. [Google Scholar] [CrossRef]
- Arık, M.; Neslihan, C. Fluorescence quenching of fluorescein with molecular oxygen in solution. J. Photochem. Photobiol. A Chem. 2005, 170, 105–111. [Google Scholar] [CrossRef]
- Grabolle, M.; Spieles, M.; Lesnyak, V.; Gaponik, N. Determination of the fluorescence quantum yield of quantum dots: Suitable procedures and achievable uncertainties. Anal. Chem. 2009, 81, 6285–6294. [Google Scholar] [CrossRef]
- Moreno-Tovar, R.; Terrés, E.; Rangel-Mendez, J.R. Oxidation and EDX elemental mapping characterization of an ordered mesoporous carbon: Pb(II) and Cd(II) removal. Appl. Surf. Sci. 2014, 303, 373–380. [Google Scholar] [CrossRef]
- Kothleitner, G.; Neish, M.J.; Lugg, N.R.; Findlay, S.D.; Grogger, W.; Hofer, F.; Allen, L.J. Quantitative elemental mapping at atomic resolution using X-ray spectroscopy. Phys. Rev. Lett. 2014, 112, 1–5. [Google Scholar] [CrossRef]
- Aydin, H.; El-Nasser, H.M.; Aydin, C.; Al-Ghamdi, A.A.; Yakuphanoglu, F. Synthesis and characterization of nanostructured undoped and Sn doped ZnO thin films via sol–gel approach. Appl. Surf. Sci. 2015, 350, 109–114. [Google Scholar] [CrossRef]
- Uddandarao, P.; Balakrishnan, R.M. Thermal and optical characterization of biologically synthesized ZnS nanoparticles synthesized from an endophytic fungus Aspergillus flavus: A colorimetric probe in metal detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 175, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Loan, T.T.; Huong, V.H.; Tham, V.T.; Long, N.N. Effect of zinc doping on the bandgap and photoluminescence of Zn2+-doped TiO2 nanowires. Phys. B Condens. Matter 2018, 532, 210–215. [Google Scholar] [CrossRef]
- Kumar, R.S.; Veeravazhuthi, V.; Muthukumarasamy, N.; Thambidurai, M. Effect of nickel doping on structural and optical properties of ZnS nanoparticles. Superlattices Microstruct. 2015, 86, 552–558. [Google Scholar] [CrossRef]
- Hunagund, S.M.; Desai, V.R.; Barretto, D.A.; Pujar, M.S.; Kadadevarmath, J.S.; Vootla, S.; Sidarai, A.H. Photocatalysis effect of a novel green synthesis gadolinium doped titanium dioxide nanoparticles on their biological activities. J. Photochem. Photobiol. A Chem. 2017, 346, 159–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Mckelvie, I.D.; Cattrall, R.W.; Kolev, S.D. Colorimetric detection based on localised surface plasmon resonance of gold nanoparticles: Merits, inherent shortcomings and future prospects. Talanta 2016, 152, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 33, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, Y.; Chakraborty, A. Label-free cysteamine-capped silver nanoparticle-based colorimetric assay for Hg(II) detection in water with subnanomolar exactitude. ACS Sustain. Chem. Eng. 2014, 2, 2149–2154. [Google Scholar] [CrossRef]
- Wang, F.; Huang, W.; Wang, Y. Fluorescence enhancement effect for the determination of curcumin with yttrium (III)–curcumin–sodium dodecyl benzene sulfonate system. J. Lumin. 2008, 128, 110–116. [Google Scholar] [CrossRef]
- Li, Z.; Ma, J.; Zong, Y.; Men, Y. ZnS nanoparticles for high-sensitive fluorescent detection of pyridine compounds. J. Alloys Compd. 2013, 559, 39–44. [Google Scholar] [CrossRef]
- Hemmateenejad, B.; Yousefinejad, S. Interaction study of human serum albumin and ZnS nanoparticles using fluorescence spectrometry. J. Mol. Struct. 2013, 1037, 317–322. [Google Scholar] [CrossRef]
- Wang, F.; Gu, Z.; Lei, W.; Wang, W.; Xia, X.; Hao, Q. Chemical graphene quantum dots as a fluorescent sensing platform for highly efficient detection of copper (II) ions. Sens. Actuators B Chem. 2014, 190, 516–522. [Google Scholar] [CrossRef]
- Poornaprakash, B.; Poojitha, P.T.; Chalapathi, U.; Subramanyam, K.; Park, S. Synthesis, structural, optical, and magnetic properties of Co doped, Sm doped and Co+Sm co-doped ZnS nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 83, 180–185. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddandarao, P.; Balakrishnan, R.M.; Ashok, A.; Swarup, S.; Sinha, P. Bioinspired ZnS:Gd Nanoparticles Synthesized from an Endophytic Fungi Aspergillus flavus for Fluorescence-Based Metal Detection. Biomimetics 2019, 4, 11. https://doi.org/10.3390/biomimetics4010011
Uddandarao P, Balakrishnan RM, Ashok A, Swarup S, Sinha P. Bioinspired ZnS:Gd Nanoparticles Synthesized from an Endophytic Fungi Aspergillus flavus for Fluorescence-Based Metal Detection. Biomimetics. 2019; 4(1):11. https://doi.org/10.3390/biomimetics4010011
Chicago/Turabian StyleUddandarao, Priyanka, Raj Mohan Balakrishnan, Apoorva Ashok, Sai Swarup, and Priti Sinha. 2019. "Bioinspired ZnS:Gd Nanoparticles Synthesized from an Endophytic Fungi Aspergillus flavus for Fluorescence-Based Metal Detection" Biomimetics 4, no. 1: 11. https://doi.org/10.3390/biomimetics4010011
APA StyleUddandarao, P., Balakrishnan, R. M., Ashok, A., Swarup, S., & Sinha, P. (2019). Bioinspired ZnS:Gd Nanoparticles Synthesized from an Endophytic Fungi Aspergillus flavus for Fluorescence-Based Metal Detection. Biomimetics, 4(1), 11. https://doi.org/10.3390/biomimetics4010011