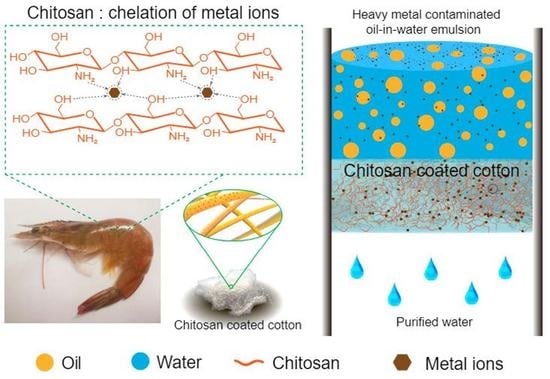
Bio-Inspired Eco-Friendly Superhydrophilic/Underwater Superoleophobic Cotton for Oil-Water Separation and Removal of Heavy Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CTS-Coated Cotton
2.2.1. Pretreatment of the Cotton
2.2.2. Preparation of CTS-Coated Cotton
2.3. Oil-Water Separation
2.3.1. Separation of Immiscible Oil-Water Mixtures
2.3.2. Separation of Heavy Metal Ion-Contaminated Emulsions
2.4. Characterization and Measurement
3. Result and Discussion
3.1. The Characterization of the CTS-Coated Cotton
3.2. The Wettability of the CTS-Coated Cotton
3.3. Separation of Oil-Water Mixtures
3.3.1. Separation of Immiscible Oil-Water Mixtures
3.3.2. Separation of Heavy Metal Ion-Contaminated Emulsions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bhushan, B. Biomimetics: Bioinspired Hierarchical-Structured Surfaces for Green Science and Technology, 3rd ed.; Springer International: New York, NY, USA, 2018. [Google Scholar]
- Clark, R.B.; Frid, C.; Attrill, M. Marine Pollution; Clarendon Press: Oxford, UK, 1989; Volume 4. [Google Scholar]
- Kokal, S. Crude-Oil Emulsions: A State-of-the-Art Review. SPE Prod. Facil. 2005, 20, 5–13. [Google Scholar] [CrossRef]
- Rao, D.G.; Senthilkumar, R.; Byrne, J.A.; Feroz, S. Wastewater Treatment: Advanced Processes and Technologies; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Bhanvase, B.A.; Ugwekar, R.P.; Mankar, R.B. Novel Water Treatment and Separation Methods: Simulation of Chemical Processes; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Prince, L. Microemulsions Theory and Practice; Elsevier: London, UK, 2012. [Google Scholar]
- Jamaly, S.; Giwa, A.; Hasan, S.W. Recent Improvements in Oily Wastewater Treatment: Progress, Challenges, and Future Opportunities. J. Environ. Sci. 2015, 37, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Song, Y.; Jiang, L. Applications of Bio-inspired Special Wettable Surfaces. Adv. Mater. 2011, 23, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Cao, M.; Fujishima, A.; Jiang, L. Bio-Inspired Titanium Dioxide Materials with Special Wettability and Their Applications. Chem. Rev. 2014, 114, 10044–10094. [Google Scholar] [CrossRef]
- Li, F.; Kong, W.; Bhushan, B.; Zhao, X.; Pan, Y. Ultraviolet-Driven Switchable Superliquiphobic/Superliquiphilic Coating for Separation of Oil-Water Mixtures and Emulsions and Water Purification. J. Colloid Interface Sci. 2019, 557, 395–407. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, Z.; Li, F.; Miao, G.; Xu, T.; Miao, X.; Song, Y.; Li, X.; Ren, G. Superlyophobic graphene oxide/polydopamine coating under liquid system for liquid/liquid separation, dye removal, and anti-corrosion. Carbon 2022, 190, 329–336. [Google Scholar] [CrossRef]
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M.; Van der Bruggen, B. Superhydrophilic and Underwater Superoleophobic Membranes—A Review of Synthesis Methods. Prog. Polym. Sci. 2019, 98, 101166. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Z.; Mai, Z.; Ma, Y.; Liu, B.; Jiang, L.; Zhu, D. A Super-Hydrophobic and Super-Oleophilic Coating Mesh Film for the Separation of Oil and Water. Angew. Chem. Int. Ed. 2004, 43, 2012–2014. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Tai, N.-H.; Lee, S.-B.; Kuo, W.-S. Superhydrophobic and Superoleophilic Properties of Graphene-Based Sponges Fabricated Using a Facile Dip Coating Method. Energy Environ. Sci. 2012, 5, 7908–7912. [Google Scholar] [CrossRef]
- Huang, J.Y.; Li, S.H.; Ge, M.Z.; Wang, L.N.; Xing, T.L.; Chen, G.Q.; Liu, X.F.; Al-Deyab, S.S.; Zhang, K.Q.; Chen, T. Robust Superhydrophobic TiO2@ Fabrics for UV Shielding, Self-Cleaning and Oil–Water Separation. J. Mater. Chem. A 2015, 3, 2825–2832. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and Superoleophilic PVDF Membranes for Effective Separation of Water-in-oil Emulsions with High Flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef]
- Gu, J.; Fan, H.; Li, C.; Caro, J.; Meng, H. Robust Superhydrophobic/Superoleophilic Wrinkled Microspherical MOF@ RGO Composites for Efficient Oil–Water Separation. Angew. Chem. 2019, 131, 5351–5355. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Pan, Y.; Zhao, X. A Facile and Effective Method to Fabricate Superhydrophobic/Superoeophilic Surface for the Separation of Both Water/Oil Mixtures and Water-in-Oil Emulsions. Polymers 2017, 9, 563. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, Z.; Xu, X.; Zhu, X.; Men, X.; Zhou, X. Superhydrophilic–Superoleophobic Coatings. J. Mater. Chem. 2012, 22, 2834–2837. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Huang, S.; Pan, Y.; Zhao, X. Flexible, Durable, and Unconditioned Superoleophobic/Superhydrophilic Surfaces for Controllable Transport and Oil–Water Separation. Adv. Funct. Mater. 2018, 28, 1706867. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, S.; Li, F.; Zhao, X.; Wang, W. Coexistence of Superhydrophilicity and Superoleophobicity: Theory, Experiments and Applications in Oil/Water Separation. J. Mater. Chem. A 2018, 6, 15057–15063. [Google Scholar] [CrossRef]
- Li, F.; Wang, S.; Zhao, X.; Shao, L.; Pan, Y. Durable Superoleophobic Janus Fabric with Oil Repellence and Anisotropic Water-Transport Integration toward Energetic-Efficient Oil–Water Separation. ACS Appl. Mater. Interfaces 2022, 14, 37170–37181. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Kong, W.; Zhao, X.; Pan, Y. Multifunctional TiO2-Based Superoleophobic/Superhydrophilic Coating for Oil–Water Separation and Oil Purification. ACS Appl. Mater. Interfaces 2020, 12, 18074–18083. [Google Scholar] [CrossRef]
- Kong, W.; Li, F.; Pan, Y.; Zhao, X. Hygro-Responsive, Photo-Decomposed Superoleophobic/Superhydrophilic Coating for On-Demand Oil–Water Separation. ACS Appl. Mater. Interfaces 2021, 13, 35142–35152. [Google Scholar] [CrossRef]
- Li, F.; Bhushan, B.; Pan, Y.; Zhao, X. Bioinspired Superoleophobic/Superhydrophilic Functionalized Cotton for Efficient Separation of Immiscible Oil-Water Mixtures and Oil-Water Emulsions. J. Colloid Interface Sci. 2019, 548, 123–130. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, S.; Lin, L.; Chen, L.; Liu, M.; Feng, L.; Jiang, L. A Novel Superhydrophilic and Underwater Superoleophobic Hydrogel-coated Mesh for Oil/Water Separation. Adv. Mater. 2011, 23, 4270–4273. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Tian, Y.; Jiang, L. Bioinspired Interfaces with Superwettability: From Materials to Chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Miao, G.; Gao, Z.; Xu, T.; Li, F.; Miao, X.; Song, Y.; Li, X.; Ren, G.; Zhu, X. Nanostructured Copper Hydroxide-Based Interfaces for Liquid/Liquid and Liquid/Gas Separations. Sep. Purif. Technol. 2022, 298, 121573. [Google Scholar] [CrossRef]
- Lin, L.; Liu, M.; Chen, L.; Chen, P.; Ma, J.; Han, D.; Jiang, L. Bio-inspired Hierarchical Macromolecule–Nanoclay Hydrogels for Robust Underwater Superoleophobicity. Adv. Mater. 2010, 22, 4826–4830. [Google Scholar] [CrossRef]
- Whitacre, D.M. Reviews of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 202. [Google Scholar]
- Bhatnagar, A.; Sillanpää, M. Applications of Chitin-and Chitosan-Derivatives for the Detoxification of Water and Wastewater—A Short Review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef]
- Yan, L.; Li, P.; Zhou, W.; Wang, Z.; Fan, X.; Chen, M.; Fang, Y.; Liu, H. Shrimp Shell-Inspired Antifouling Chitin Nanofibrous Membrane for Efficient Oil/Water Emulsion Separation with in Situ Removal of Heavy Metal Ions. ACS Sustain. Chem. Eng. 2018, 7, 2064–2072. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, F.; Tao, L.; Liu, N.; Gao, C.; Feng, L.; Wei, Y. Bio-Inspired Anti-Oil-Fouling Chitosan-Coated Mesh for Oil/Water Separation Suitable for Broad PH Range and Hyper-Saline Environments. ACS Appl. Mater. Interfaces 2013, 5, 11971–11976. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. A Review and Experimental Verification of Using Chitosan and Its Derivatives as Adsorbents for Selected Heavy Metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef]
- Apte, S.K.; Naik, S.D.; Sonawane, R.S.; Kale, B.B.; Baeg, J.O. Synthesis of Nanosize-necked Structure A-and γ-Fe2O3 and Its Photocatalytic Activity. J. Am. Ceram. Soc. 2007, 90, 412–414. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Hong, K.-J.; Kajiuchi, T.; Yang, J.-W. Synthesis of Chitosan-Based Polymeric Surfactants and Their Adsorption Properties for Heavy Metals and Fatty Acids. Int. J. Biol. Macromol. 2005, 36, 152–158. [Google Scholar] [CrossRef]
- Ngah, W.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of Dyes and Heavy Metal Ions by Chitosan Composites: A Review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Cheng, Q.; Wang, C.; Li, H.; Han, X.; Fan, Z.; Su, G.; Pan, D.; Li, Z. Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: A review. Chemosphere 2021, 279, 130927. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Du, Y.; Li, Y.; Wu, T. A superhydrophilic and underwater superoleophobic chitosan–TiO2 composite membrane for fast oil-in-water emulsion separation. RSC Adv. 2017, 7, 41838–41846. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Lü, T.; Qi, D.; Cao, Z.; Zhang, D.; Zhao, H. Synthesis of quaternized chitosan-coated magnetic nanoparticles for oil-water separation. Mater. Lett. 2016, 191, 128–131. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, J.; Ge, S.; Jiang, C.; Guo, T.; Peng, T.; Huang, T.; Xie, L. Biocompatible, hydrophobic and resilience graphene/chitosan composite aerogel for efficient oil−water separation. Surf. Coat. Tech. 2020, 385, 125361. [Google Scholar] [CrossRef]
- Wang, C.; He, G.; Cao, J.; Fan, L.; Cai, W.; Yin, Y. Underwater Superoleophobic and Salt-Tolerant Sodium Alginate/N-Succinyl Chitosan Composite Aerogel for Highly Efficient Oil–Water Separation. ACS Appl. Polym. Mater. 2020, 2, 1124–1133. [Google Scholar] [CrossRef]
- Rumble, J.R. CRC Handbook of Chemistry and Physics, 99th ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Mourya, V.K.; Inamdar, N.N.; Tiwari, A. Carboxymethyl Chitosan and Its Applications. Adv. Mater. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Wetting Behavior of Water and Oil Droplets in Three-Phase Interfaces for Hydrophobicity/Philicity and Oleophobicity/Philicity. Langmuir 2009, 25, 14165–14173. [Google Scholar] [CrossRef]
- De Gennes, P.-G.; Brochard-Wyart, F.; Quéré, D. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves; Springer: Berlin/Heidelberg, Germany, 2004; Volume 315. [Google Scholar]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Wang, J.; Wang, Z.; Ji, D.; Wang, S.; Wei, P.; Cao, W. Bio-Inspired Eco-Friendly Superhydrophilic/Underwater Superoleophobic Cotton for Oil-Water Separation and Removal of Heavy Metals. Biomimetics 2022, 7, 177. https://doi.org/10.3390/biomimetics7040177
Li F, Wang J, Wang Z, Ji D, Wang S, Wei P, Cao W. Bio-Inspired Eco-Friendly Superhydrophilic/Underwater Superoleophobic Cotton for Oil-Water Separation and Removal of Heavy Metals. Biomimetics. 2022; 7(4):177. https://doi.org/10.3390/biomimetics7040177
Chicago/Turabian StyleLi, Feiran, Jian Wang, Zhuochao Wang, Dongchao Ji, Shuai Wang, Pengcheng Wei, and Wenxin Cao. 2022. "Bio-Inspired Eco-Friendly Superhydrophilic/Underwater Superoleophobic Cotton for Oil-Water Separation and Removal of Heavy Metals" Biomimetics 7, no. 4: 177. https://doi.org/10.3390/biomimetics7040177
APA StyleLi, F., Wang, J., Wang, Z., Ji, D., Wang, S., Wei, P., & Cao, W. (2022). Bio-Inspired Eco-Friendly Superhydrophilic/Underwater Superoleophobic Cotton for Oil-Water Separation and Removal of Heavy Metals. Biomimetics, 7(4), 177. https://doi.org/10.3390/biomimetics7040177