Paramyxovirus Infections in Ex Vivo Lung Slice Cultures of Different Host Species
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- Hydroxyethylagarose, Type VII, low gelling temperature (Sigma-Aldrich, St. Louis, MO, USA; Cat. no.: A4018);
- Scissors/forceps/clamp;
- 10 mL syringe (Fisher Scientific, Hampton, NH, USA; Cat. no.: NC9001097);
- Catheter (20G I.V.1 in 25 mm, Patterson Veterinary, Saint Paul, MN, USA; Cat.no.: 32058-46-20);
- Blunt-end needles (Miltenyi Biotec, Bergisch Gladbach, Germany; Cat. no.: 130-091-558);
- Petri dishes, 150 × 15 mm (Fisher Scientific, Hampton, NH, USA; Cat. no.: 08-757-148);
- Cutting board;
- Microtome blade (MX35 Ultra, Thermo Scientific, Waltham, MA, USA);
- Six-, 12-, 24- or 48-wells plates (Greiner, Kremsmunster, Austria);
- Thin-bottom/glass-bottom dishes (e.g., Ibidi, Planegg, Germany; Cat. no.: 81156);
- Fifteen-milliliter tubes (Greiner, Kremsmunster, Austria);
- V-bottom 96-wells plate (Greiner, Kremsmunster, Austria);
- Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA; Cat. no.: T8787);
- 10% buffered formalin (Sigma-Aldrich, St. Louis, MO, USA; Cat. no.: HT501128);
- Hank’s Buffered Saline Solution (HBSS; Lonza, Basel, Switzerland; Cat. no.: BE10-508F);
- Collagenase (Invitrogen, Carlsbad, CA, USA, Cat. no.: 17100-017);
- DNAse (Sigma-Aldrich, St. Louis, MO, USA; Cat. no.: 10104159);
- Cell strainers (100 μm nylon; Becton Dickinson, NJ, USA);
- Red blood cell lysis buffer (Roche, Basel, Switzerland; Cat. no.: 11814389001);
- Dulbecco’s phosphate-buffered saline (DPBS; Invitrogen, Carlsbad, CA, USA; Cat. no.: 14190-250);
- Dulbecco’s modified Eagle’s medium (DMEM; Lonza Bio-Whittaker, Basel, Switserland);
- Ham’s Nutrient Mixture F12 powder (Invitrogen, Carlsbad, CA, USA; Cat. no.: 12500062);
- Glutamax (Invitrogen, Carlsbad, CA, USA; Cat. no.: 35050-061);
- Fetal bovine serum (FBS; Lonza Bio-Whittaker, Basel, Switserland; Cat. no.: 14-501GM);
- Penicillin (100 U/mL)/Streptomycin (100 mg/mL) (Lonza Bio-Whittaker, Basel, Switserland; Cat. No.: DE17-602E);
- Amphotericin (1 μg/mL) (Fungizone, Sigma-Aldrich, St. Louis, MO, USA; A4888);
- Bovine serum albumin (BSA; Sigma, St. Louis, MO, USA; Cat. no.: A1595);
- Ethylenediaminetetraacetic acid (EDTA; Sigma, St. Louis, MO, USA; Cat. no.: ED2SS);
- Paraformaldehyde (PFA; Sigma-Aldrich, St. Louis, MO, USA; Cat. no.: 441244).
2.2. Equipment
- Phase-contrast inverted light microscope;
- Macroscopic imaging lamp and emission filter;
- Inverted fluorescence microscope;
- Confocal laser-scanning microscope;
- Flow cytometer.
3. Procedure
3.1. Inflation of Lungs. Time for Completion: 1 h
- Prepare low-melting point agarose beforehand.
CRITICAL STEP Prepare 4% (w/v) low-melting point agarose by dissolving agarose in phosphate-buffered saline (PBS) and boil for several minutes (e.g., using a microwave oven, be careful for boiling over). As an alternative, agarose can be prepared by autoclaving. Be sure to allow agarose to cool down to 42 °C (e.g., in a water bath) before inflating the lungs.
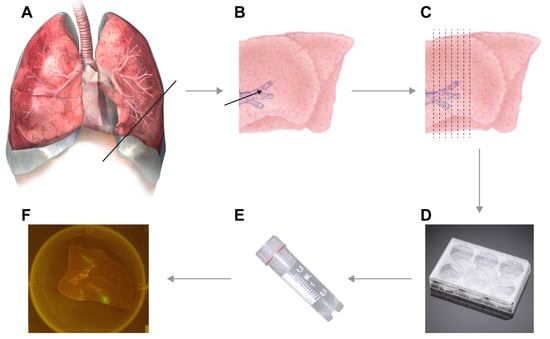
NOTE: The volume of low-melting point agarose should be adjusted according to the size of the lungs. - Resect the lungs, including the distal part of the trachea, from the experimental animal.
CRITICAL STEP Resection should be performed within 6 h after euthanasia, preferably sooner.
CRITICAL STEP Be sure not to damage the lungs while resecting, as this will interfere with the inflation process.
- Locate the trachea (for small species) or primary bronchus (for larger species) through which you wish to inflate.NOTE: For smaller mammals, inflation through the trachea works best, but for larger animals (body weight > 3–5 kg) inflation through the primary bronchus is more efficient.
- Fill a syringe with a 42 °C pre-warmed 1:1 mixture of 4% (w/v) low-melting point agarose and lung slice medium (DMEM/Ham’s F12 medium supplemented with 10% (v/v) FBS, penicillin, streptomycin, l-glutamine and amphotericin B). Final agarose percentage is 2% (w/v).
CRITICAL STEP Avoid the formation of air bubbles in the agarose.
NOTE: Decide on the size of the syringe dependent on the size of the trachea or bronchus through which you will inflate. Either the pipette tip, blunt-end needles, catheters, cannulas the end of the syringe should be a tight fit into the trachea or bronchus. - Insert the end of the syringe into the trachea or primary bronchus, enforce placement inside the trachea or bronchus by clamping (or use sutures as an alternative) the trachea or bronchus around the needle.
CRITICAL STEP Be careful not to damage the trachea or bronchus while inserting the needle into the trachea or bronchus. We advise the use of pipette tips, blunt-end needles, catheters or cannulas instead of sharp needles at the end of the syringe.
- Inject the proper amount of agarose into the lungs. Check inflation while injecting; keep adding agarose until the lungs are completely inflated (see Video S1).
CRITICAL STEP It is not always easy to determine the best level of inflation. In our experience, the volume used for inflation should be slightly higher than the tidal volume of the animal, which is usually around 7 mL per kg body weight. If you are inflating just one lobe of the lung, the volume should be adapted accordingly.
- Remove needle but keep the clamp positioned on the trachea or bronchus and allow the inflated lungs to solidify on ice (5 min for smaller species and 10–30 min for larger species).
3.2. Preparation of Lung Slices. Time for Completion: 1 h
- Transfer lungs to biosafety cabinet, remove the clamp, prepare a surface for cutting (cutting board or Petri dish) and microtome blade. Prepare the lungs for slicing by finding anatomically interesting locations.
CRITICAL STEP Dependent on the focus of the experiment, you have to decide which part is anatomically interesting. Decide on whether you want to use for example the left or right side, and either the upper or lower parts of available lobes. To check viability, we advise to include a cut of the primary bronchus in the slice, to determine the beating of cilia indicating viable epithelial cells.
- Start with an initial cut to ensure that a straight edge is available for cutting. Now manually prepare slices of approximately 1 mm thickness. If desired, the lungs can be attached to the cutting board by needles (see Video S2).NOTE: As a rule of thumb, slices are thin enough for culture if you can see through the slice while cutting it and actually see the microtome blade.
- Gently transfer cut slices into 6-, 12-, 24-, or 48-well plates (dependent on size of the slices) that were pre-filled with lung slice culture medium.
- Infection of slices can be performed immediately.
PAUSE STEP Slices can also be infected after 24 h of culture at 37 °C in 5% (v/v) CO2. We have never observed discrepancies between obtained results after either direct infection or after overnight culture.
3.3. Infection of Lung Slices. Time for Completion: 2 h
- Transfer the lung slices into empty 6-, 12-, 24-, or 48-well plates (dependent on size of the slices).
- Gently add virus (preferably a recombinant virus expressing a fluorescent reporter protein) in a drop wise fashion onto the lung slice. Typically, we used an inoculation volume of 150 μL; always include a mock infection as negative control.NOTE: We typically use 1–5 × 105 Tissue Culture Infectious Dose-50 (TCID50) per slice; variations are possible.
- Incubate at 37 °C in 5% (v/v) CO2 for 1 h.
- After 1 h, add an appropriate volume of lung slice medium (inoculum can be washed away) and return plates to 37 °C in 5% (v/v) CO2.
- In our experience, slices can be held viable (based on beating cilia in epithelium) and followed in time for a maximum of 7 days. Culture medium has to be replaced to ensure viability every other day (i.e., day 2, 4 and 6).
CRITICAL STEP When replacing culture medium, medium should be taken off and directly replaced, not allowing the lung slices to dehydrate.
NOTE: Beating of cilia is the best measure for viability, however when beating of cilia is no longer observed slices start disintegrating. Depending on the goal of the experiment, non-viable slices should be removed or kept in culture.
3.4. Follow-Up in Time (Viability). Time for Completion: Up to 7 Days
- Slices should be checked for viability on daily basis: find a bronchus with cilia under a normal light microscope. Movement (see Video S3) of cilia is indicative of viable epithelial cells, suggesting there are live cells in culture.
3.5. Follow-Up in Time (Macroscopic and Microscopic Fluorescence). Time for Completion: Up to 7 Days
- If fluorescent reporter viruses were used, slices should be checked for fluorescence on a daily basis (see Video S4 and Figure 2).
- Use a macroscopic imaging lamp as described previously [7] in combination with an emission filter to visualize fluorescence macroscopically. Shine lamp directly on lung slices and find fluorescent spots. Photographs can be acquired if desired.
- Use an inverted UV microscope or confocal laser scanning microscope to identify fluorescent-positive cells.
3.6. Follow-Up in Time (Flow Cytometry). Time for Completion: Up to 7 Days
- During infection and culture, effluent cells can be harvested and checked for expression of fluorescent proteins by flow cytometry if desired.
- Harvest supernatants from slices (e.g., on day 2, 4 and 6 while replacing culture medium), transfer supernatant to tubes and centrifuge (5–10 min, 300× g).
- Transfer pellets to 96-wells V-bottom plate, wash once in FACS buffer (PBS + 0.5% (v/v) BSA + 2 mM EDTA).
- OPTIONAL STEP To determine which subsets of cells are infected, a fluorescence activated cell sorter (FACS) staining for specific cell types can be performed at this point.NOTE: Cells that migrate out of the lung slices are mainly lymphoid, myeloid or epithelial in origin. To study the tropism of infection you can selectively stain for example T-cells, B-cells, natural killer (NK) cells, neutrophils, granulocytes, dendritic cells, macrophages or epithelial cells, or combinations thereof.
- Acquire cells on flow cytometer to determine number of virus-positive cells.
3.7. End of Experiment
3.7.1. End of Experiment—Confocal Laser Scanning Microscopy. Time for Completion: 2 h
- Fix lung slices by transferring slices into new plates pre-filled with 4% (w/v) paraformaldehyde (PFA) in PBS.
CRITICAL STEP Not all fluorophores are well-preserved by PFA fixation. Sensitivity of fluorophores to fixation should be tested beforehand, and the fixative of mounting medium can be adjusted accordingly.
- Wash slices after 10 min and permeabilize in 0.1% (v/v) Triton X-100 for 30 min.
- Wash slices and subsequently stain with fluorescent antibodies of choice.
CRITICAL STEP To understand tissue morphology, it is important to counterstain nuclei (for example with TO-PRO-3 or NucBlue) and add a marker of choice. We found it useful to stain cilia using an antibody against class IV β-tubulin.
- Transfer slices to thin-bottom/glass-bottom dishes.
- View fluorescence by confocal laser scanning microscopy (using an inverted microscope, see Figure 3).NOTE: Since slices are approximately 1-mm thick, z-stacks can be generated and from those 3D images can be rendered.
3.7.2. End of Experiment—Immunohistochemistry. Time for Completion: 3 Days
- Fix lung slices by transferring slices into new plates pre-filled with 10% (v/v) formalin or directly boxing slices into cassettes kept in formalin, leave overnight and continue with paraffin embedding for immunohistochemistry.
3.7.3. End of Experiment—Single Cell Flow Cytometry. Time for Completion: 3 h
- Wash slices twice in Hank’s balanced salt solution (HBSS).
- Transfer slices into petri dishes and manually cut in small pieces.
- Transfer pieces into 6-well plate pre-filled with HBSS + collagenase (300 units/mL) + DNAse (0.15 mg/mL).
- Incubate for 1 h on rocking platform at 37 °C in 5% (v/v) CO2.
- Prepare single cell suspension by straining small pieces over 100 μm cell strainer.
- Centrifuge and lyse red blood cells by adding 3 mL red blood cell lysis buffer to pellet, incubate 37 °C in 5% (v/v) CO2 for three minutes.
- Centrifuge, resuspend pellet in FACS buffer and perform FACS staining of choice.
3.8. Ex Vivo Lung Slice Analysis of In Vivo Infected Animals
4. Expected Results
5. Reagents Setup
- 4% agarose: prepare correct weight of hydroxyethylagarose in DPBS;
- Lung slice medium: DMEM/Ham’s Nutrient Mixture F12 powder, Glutamax, FBS, Penicillin/Streptomycin, amphotericin;
- Fluorescence activated cell sorter buffer: PBS + 0.5% (v/v) BSA + 2 mM EDTA (use stock of 0.5 M in distilled water);
- Paraformaldehyde: dissolve appropriate weight in PBS with high pH by initially supplementing with NaOH. After PFA has completely dissolved, pH should be re-adjusted to 7.4. Always prepare fresh or store aliquoted at −20 °C. Do not use buffered formalin when directly screening fluorescence, as this will disrupt the fluorescence of most reporter proteins.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bergner, A.; Sanderson, M.J. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J. Gen. Physiol. 2002, 119, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Dandurand, R.J.; Wang, C.G.; Phillips, N.C.; Eidelman, D.H. Responsiveness of individual airways to methacholine in adult rat lung explants. J. Appl. Physiol. 1993, 75, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Lemon, K.; de Vries, R.D.; Mesman, A.W.; McQuaid, S.; van Amerongen, G.; Yuksel, S.; Ludlow, M.; Rennick, L.J.; Kuiken, T.; Rima, B.K.; et al. Early target cells of measles virus after aerosol infection of non-human primates. PLoS Pathog. 2011, 7, e1001263. [Google Scholar] [CrossRef] [PubMed]
- Mesman, A.W.; de Vries, R.D.; McQuaid, S.; Duprex, W.P.; de Swart, R.L.; Geijtenbeek, T.B. A prominent role for DC-SIGN+ dendritic cells in initiation and dissemination of measles virus infection in non-human primates. PLoS ONE 2012, 7, e49573. [Google Scholar] [CrossRef] [PubMed]
- Meunier, I.; von Messling, V. PB1-F2 modulates early host responses but does not affect the pathogenesis of H1N1 seasonal influenza virus. J. Virol. 2012, 86, 4271–4278. [Google Scholar] [CrossRef] [PubMed]
- Siminski, J.T.; Kavanagh, T.J.; Chi, E.; Raghu, G. Long-term maintenance of mature pulmonary parenchyma cultured in serum-free conditions. Am. J. Physiol. 1992, 262, L105–L110. [Google Scholar] [CrossRef] [PubMed]
- De Swart, R.L.; Ludlow, M.; de Witte, L.; Yanagi, Y.; van Amerongen, G.; McQuaid, S.; Yuksel, S.; Geijtenbeek, T.B.; Duprex, W.P.; Osterhaus, A.D. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 2007, 3, e178. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, M.; Herfst, S.; Schrauwen, E.J.; van den Hoogen, B.G.; Osterhaus, A.D.; Fouchier, R.A. An improved plaque reduction virus neutralization assay for human metapneumovirus. J. Virol. Methods 2007, 143, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Duprex, W.P.; McQuaid, S.; Hangartner, L.; Billeter, M.A.; Rima, B.K. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J. Virol. 1999, 73, 9568–9575. [Google Scholar] [PubMed]
- Nguyen, D.T.; de Vries, R.D.; Ludlow, M.; van den Hoogen, B.G.; Lemon, K.; van Amerongen, G.; Osterhaus, A.D.; de Swart, R.L.; Duprex, W.P. Paramyxovirus infections in ex vivo lung slice cultures of different host species. J. Virol. Methods 2013, 193, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Haagmans, B.L.; van den Brand, J.M.; Provacia, L.B.; Raj, V.S.; Stittelaar, K.J.; Getu, S.; de Waal, L.; Bestebroer, T.M.; van Amerongen, G.; Verjans, G.M.; et al. Asymptomatic Middle East respiratory syndrome coronavirus infection in rabbits. J. Virol. 2015, 89, 6131–6135. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vries, R.D.; Rennick, L.J.; Duprex, W.P.; De Swart, R.L. Paramyxovirus Infections in Ex Vivo Lung Slice Cultures of Different Host Species. Methods Protoc. 2018, 1, 12. https://doi.org/10.3390/mps1020012
De Vries RD, Rennick LJ, Duprex WP, De Swart RL. Paramyxovirus Infections in Ex Vivo Lung Slice Cultures of Different Host Species. Methods and Protocols. 2018; 1(2):12. https://doi.org/10.3390/mps1020012
Chicago/Turabian StyleDe Vries, Rory D., Linda J. Rennick, W. Paul Duprex, and Rik L. De Swart. 2018. "Paramyxovirus Infections in Ex Vivo Lung Slice Cultures of Different Host Species" Methods and Protocols 1, no. 2: 12. https://doi.org/10.3390/mps1020012
APA StyleDe Vries, R. D., Rennick, L. J., Duprex, W. P., & De Swart, R. L. (2018). Paramyxovirus Infections in Ex Vivo Lung Slice Cultures of Different Host Species. Methods and Protocols, 1(2), 12. https://doi.org/10.3390/mps1020012