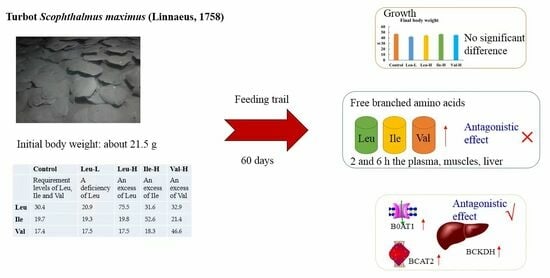
Response of Turbot Scophthalmus maximus (Linnaeus, 1758) to Imbalanced Branched-Chain Amino Acids in Diets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Fish and Feeding Trial
2.3. Sample Collection and Growth Performance Indices
2.4. Analytical Methods
2.4.1. Proximate Composition and Amino Acid Concentrations
2.4.2. Gene Expression Analysis
2.5. Statistical Analysis
3. Results
3.1. Growth Performance and Whole-Body Proximate Composition
3.2. Free BCAA Concentrations of Plasma, Muscle and Liver Samples
3.3. Gene Expression of Amino Acid and Peptide Transporters
3.4. Related Gene Expression of BCAA Catabolism
3.5. The Expression of Genes Related to mTOR Pathway in Muscles and Livers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castillo, S.; Gatlin, D.M. Dietary requirements for leucine, isoleucine and valine (branched-chain amino acids) by juvenile red drum Sciaenops ocellatus. Aquac. Nutr. 2018, 24, 1056–1065. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, I.; Fatma, S.; Peres, H. Role of branched-chain amino acids on growth, physiology and metabolism of different fish species: A review. Aquac. Nutr. 2021, 27, 1270–1289. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Jiang, Z.; Sun, M.; Si, B.; Chen, F.; Bao, N. Interactive effects of dietary leucine and isoleucine on growth, blood parameters, and amino acid profile of Japanese flounder Paralichthys olivaceus. Fish Physiol. Biochem. 2017, 43, 1265–1278. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Huang, Z.; Wang, J.; Wang, Y.; Yu, W. Effects of dietary leucine levels on intestinal antioxidant status and immune response for juvenile golden pompano (Trachinotus ovatus) involved in Nrf2 and NF-κB signaling pathway. Fish Shellfish Immunol. 2020, 107, 336–345. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, X.; Wu, X.; Gao, Y.; Li, X.; Dong, Y.; Yao, W. Effects of dietary leucine levels on growth, feed utilization, neuro-endocrine growth axis and TOR-related signaling genes expression of juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Aquaculture 2019, 504, 172–181. [Google Scholar] [CrossRef]
- Zou, T.; Cao, S.; Xu, W.; Han, D.; Liu, H.; Yang, Y.; Jin, J.; Zhu, X.; Xie, S. Effects of dietary leucine levels on growth, tissue protein content and relative expression of genes related to protein synthesis in juvenile gibel carp (Carassius auratus gibelio var. CAS III). Aquac. Res. 2018, 49, 2240–2248. [Google Scholar] [CrossRef]
- Furuya, W.M.; Cruz, T.P.D.; Gatlin III, D.M. Amino acid requirements for Nile tilapia: An update. Animals 2023, 13, 900. [Google Scholar] [CrossRef]
- Cemin, H.S.; Tokach, M.D.; Woodworth, J.C.; Dritz, S.S.; DeRouchey, J.M.; Goodband, R.D. Branched-chain amino acid interactions in growing pig diets. Transl. Anim. Sci. 2019, 3, 1246–1253. [Google Scholar] [CrossRef]
- Zhang, Z.; Monleon, D.; Verhamme, P.; Staessen, J.A. Branched-chain amino acids as critical switches in health and disease. Hypertension 2018, 72, 1012–1022. [Google Scholar] [CrossRef]
- Meyer, F.; Van Rensburg, C.J.; Gous, R.M. The response of weaned piglets to dietary valine and leucine. Animal 2017, 11, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Wiltafsky, M.K.; Pfaffl, M.W.; Roth, F.X. The effects of branched-chain amino acid interactions on growth performance, blood metabolites, enzyme kinetics and transcriptomics in weaned pigs. Br. J. Nutr. 2010, 103, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Castillo, S.; Gatlin, D.M. Imbalanced dietary levels of branched-chain amino acids affect growth performance and amino acid utilization of juvenile red drum Sciaenops ocellatus. Aquaculture 2018, 497, 17–23. [Google Scholar] [CrossRef]
- Robinson, E.H.; Poe, W.E.; Wilson, R.P. Effects of feeding diets containing an imbalance of branched-chain amino acids on fingerling channel catfish. Aquaculture 1984, 37, 51–62. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, Z.; Duan, M.; Ma, Q.; Xu, H.; Liang, M. Responses to Graded Levels of Leucine and Branched-Chain Amino Acid Imbalance in Tiger Puffer Takifugu rubripes. Aquaculture 2022, 548, 737699. [Google Scholar] [CrossRef]
- Han, Y.; Han, R.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Gao, J. Interactive effects of dietary valine and leucine on two sizes of Japanese flounder Paralichthys olivaceus. Aquaculture 2014, 432, 130–138. [Google Scholar] [CrossRef]
- Prado, F.D.; Vera, M.; Hermida, M.; Blanco, A.; Bouza, C.; Maes, G.E.; Volckaert, F.A.M.; Martinez, P. Consortium AquaTrace. Tracing the genetic impact of farmed turbot Scophthalmus maximus on wild populations. Aquacult. Environ. Interac. 2018, 10, 447–463. [Google Scholar] [CrossRef]
- Ma, A.; Huang, Z.; Wang, X.; Xu, Y.; Guo, X. Identification of quantitative trait loci associated with upper temperature tolerance in turbot, Scophthalmus maximus. Sci. Rep. 2021, 11, 21920. [Google Scholar] [CrossRef]
- Kaushik, S.J. Whole body amino acid composition of European seabass (Dicentrarchus labrax), Gilthead seabream (Sparus aurata) and Turbot (Psetta maxima) with an estimation of their IAA requirement profiles. Aquat. Living Resour. 1998, 11, 355–358. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shima, T.; Furuita, H. Antagonistic effects of branched-chain amino acids induced by excess protein-bound leucine in diets of rainbow trout (Oncorhynchus mykiss). Aquaculture 2004, 232, 539–550. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; AOAC: Arlington, VA, USA, 2016. [Google Scholar]
- Wei, Y.; Liang, M.; Xu, H.; Zheng, K. Taurine alone or in combination with fish protein hydrolysate affects growth performance, taurine transport and metabolism in juvenile turbot (Scophthalmus maximus L.). Aquac. Nutr. 2019, 25, 396–405. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kwon, W.B.; Soto, J.A.; Stein, H.H. Effects on nitrogen balance and metabolism of branched-chain amino acids by growing pigs of supplementing isoleucine and valine to diets with adequate or excess concentrations of dietary leucine. J. Anim. Sci. 2020, 98, 1–10. [Google Scholar] [CrossRef]
- Wang, M.; Huang, Y.; Liu, W.; Xiao, K.; Wang, X.; Guo, H.; Zhang, Y.; Fan, J.; Li, X.; Jiang, G. Interactive effects of dietary leucine and isoleucine affect amino acid profile and metabolism through AKT/TOR signaling pathways in blunt snout bream (Megalobrama amblycephala). Fish Physiol. Biochem. 2022, 1–17. [Google Scholar] [CrossRef]
- Zehra, S.; Khan, M.A. Dietary leucine requirement of fingerling Catla catla (Hamilton) based on growth, feed conversion ratio, RNA/DNA ratio, leucine gain, blood indices and carcass composition. Aquacult. Int. 2015, 23, 577–595. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nakamura, K.; Matsumoto, H.; Sakai, R.; Kuwahara, T.; Kadota, Y.; Kitaura, Y.; Sato, J.; Shimomura, Y. Bolus ingestion of individual branched-chain amino acids alters plasma amino acid profiles in young healthy men. Springerplus 2014, 3, 35. [Google Scholar] [CrossRef]
- Block, K.P.; Harper, A.E. Valine metabolism in vivo: Effects of high dietary levels of leucine and isoleucine. Metabolism 1984, 33, 559–566. [Google Scholar] [CrossRef]
- May, R.C.; Piepenbrock, N.; Kelly, R.A.; Mitch, W.E. Leucine-induced amino acid antagonism in rats: Muscle valine metabolism and growth impairment. J. Nutr. 1991, 121, 293–301. [Google Scholar] [CrossRef]
- Choo, P.; Smith, T.; Cho, C.; Ferguson, H. Dietary excesses of leucine influence growth and body composition of rainbow trout. J. Nutr. 1991, 121, 1932–1939. [Google Scholar] [CrossRef]
- Ambardekar, A.A.; Reigh, R.C.; Williams, M.B. Absorption of amino acids from intact dietary proteins and purified amino acid supplements follows different time-courses in channel catfish (Ictalurus punctatus). Aquaculture 2009, 291, 179–187. [Google Scholar] [CrossRef]
- Jando, J.; Camargo, S.M.; Herzog, B.; Verrey, F. Expression and regulation of the neutral amino acid transporter B0AT1 in rat small intestine. PLoS ONE 2017, 12, e0184845. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Vanzant, E.S.; Harmon, D.L.; McLeod, K.R.; Boling, J.A.; Matthews, J.C. Ruminal and abomasal starch hydrolysate infusions selectively decrease the expression of cationic amino acid transporter mRNA by small intestinal epithelia of forage-fed beef steers. J. Dairy Sci. 2009, 92, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Barrera, M.A.; Araiza, A.B.; Zijlstra, R.T.; Bernal, H.; Cervantes, M. Effect of excess levels of lysine and leucine in wheat-based, amino acid-fortifed diets on the mRNA expression of two selected cationic amino acid transporters in pigs. J. Anim. Physiol. Anim. Nutr. 2013, 97, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, M.G.; Roshanbin, S.; Löfqvist, E.; Hellsten, S.V.; Nilsson, V.C.; Todkar, A.; Zhu, Y.; Stephansson, O.; Drgonova, J.; Uhl, G.R.; et al. B0AT2 (SLC6A15) is localized to neurons and astrocytes, and is involved in mediating the effect of leucine in the brain. PLoS ONE 2013, 8, e58651. [Google Scholar] [CrossRef] [PubMed]
- Guetg, A.; Mariotta, L.; Bock, L.; Herzog, B.; Fingerhut, R.; Camargo, S.M.; Verrey, F. Essential amino acid transporter Lat4 (Slc43a2) is required for mouse development. J. Physiol. 2015, 593, 1273–1289. [Google Scholar] [CrossRef]
- Verri, T.; Barca, A.; Pisani, P.; Piccinni, B.; Storelli, C.; Romano, A. Di-and tripeptide transport in vertebrates: The contribution of teleost fish models. J. Comp. Physiol. B 2017, 187, 395–462. [Google Scholar] [CrossRef] [PubMed]
- Javed, K.; Bröer, S. Mice lacking the intestinal and renal neutral amino acid transporter SLC6A19 demonstrate the relationship between dietary protein intake and amino acid malabsorption. Nutrients 2019, 11, 2024. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Branched-chain amino acids: Enzyme and substrate regulation. J. Nutr. 2006, 136, 207S–211S. [Google Scholar] [CrossRef]
- Dimou, A.; Tsimihodimos, V.; Bairaktari, E. The critical role of the branched chain amino acids (BCAAs) catabolism-regulating enzymes, branched-chain aminotransferase (BCAT) and branched-chain α-keto acid dehydrogenase (BCKD), in human pathophysiology. Int. J. Mol. Sci. 2022, 23, 4022. [Google Scholar] [CrossRef]
- Comesana, S.; Velasco, C.; Conde-Sieira, M.; Míguez, J.M.; Soengas, J.L.; Morais, S. Feeding stimulation ability and central effects of intraperitoneal treatment of L-leucine, L-valine, and L-proline on amino acid sensing systems in rainbow trout: Implication in food intake control. Front. Physiol. 2018, 9, 1209. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, X.; Wang, C.; Yuan, M.; Piao, X. Effects of dietary valine: Lysine ratio on the performance, amino acid composition of tissues and mRNA expression of genes involved in branched-chain amino acid metabolism of weaned piglets. Asian Austral. J. Anim. 2018, 31, 106. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Habte-Tsion, H.M.; Liu, B.; Miao, L.; Ge, X.; Xie, J.; Liang, H.; Zhou, Q.; Pan, L. Dietary leucine level affects growth performance, whole body composition, plasma parameters and relative expression of TOR and TNF-α in juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 2015, 448, 162–168. [Google Scholar] [CrossRef]
- Ren, M.; Habte-Tsion, H.M.; Liu, B.; Miao, L.; Ge, X.; Xie, J.; Zhou, Q. Dietary isoleucine requirement of juvenile blunt snout bream, Megalobrama amblycephala. Aquacult. Nutr. 2017, 23, 322–330. [Google Scholar] [CrossRef]
- Feng, L.; Gan, L.; Jiang, W.; Wu, P.; Liu, Y.; Jiang, J.; Zhou, X. Gill structural integrity changes in fish deficient or excessive in dietary isoleucine: Towards the modulation of tight junction protein, inflammation, apoptosis and antioxidant defense via NF-κB, TOR and Nrf2 signaling pathways. Fish Shellfish Immun. 2017, 63, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Garcia de la Serrana, D.; Mareco, E.A.; Vieira, V.L.; Power, D.M.; Johnston, I.A. Comparison of the transcriptional responses of skeletal muscle and bone to a flooding dose of leucine in the gilthead sea bream (Sparus aurata). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 199, 50–57. [Google Scholar] [CrossRef] [PubMed]



| Ingredient | Control | Leu-L | Leu-H | Ile-H | Val-H |
|---|---|---|---|---|---|
| Fish meal | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Peanut meal | 400.0 | 400.0 | 400.0 | 400.0 | 400.0 |
| Gelatin | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Wheat meal | 180.0 | 180.0 | 180.0 | 180.0 | 180.0 |
| Amino acid premix 1 | 85.2 | 85.2 | 85.2 | 85.2 | 85.2 |
| Fish oil | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 |
| Lecithin | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Vitamin premix 2 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Mineral premix 3 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Vitamin C | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Ca(H2PO4)2 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Choline chloride | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| DMPT 4 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Calcium propionate | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Ethoxyquin | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Leucine | 10.5 | 0.0 | 62.9 | 10.5 | 10.5 |
| Isoleucine | 0.0 | 0.0 | 0.0 | 43.9 | 0.0 |
| Valine | 0.0 | 0.0 | 0.0 | 0.0 | 34.0 |
| Glutamic acid | 26.4 | 31.7 | 0.2 | 4.5 | 9.4 |
| Glycine | 26.4 | 31.6 | 0.2 | 4.4 | 9.4 |
| Proximate composition | |||||
| Moisture | 92.0 | 76.0 | 76.1 | 62.5 | 76.9 |
| Ash | 60.9 | 62.0 | 61.9 | 61.9 | 62.3 |
| Crude protein | 505.8 | 510.7 | 503.0 | 494.1 | 502.3 |
| Crude lipid | 112.5 | 113.3 | 122.3 | 116.0 | 114.3 |
| Control | Leu-L | Leu-H | Ile-H | Val-H | |
|---|---|---|---|---|---|
| Essential amino acid | |||||
| Threonine | 14.2 | 14.9 | 14.8 | 14.4 | 15.6 |
| Valine | 17.4 | 17.5 | 17.5 | 18.3 | 46.6 |
| Methionine | 14.2 | 14.4 | 14.7 | 14.1 | 15.0 |
| Isoleucine | 19.7 | 19.3 | 19.8 | 52.6 | 21.4 |
| Leucine | 30.4 | 20.9 | 75.5 | 31.6 | 32.9 |
| Phenylalanine | 17.1 | 16.3 | 17.6 | 18.2 | 20.5 |
| Lysine | 26.4 | 26.0 | 26.4 | 26.8 | 28.5 |
| Histidine | 8.6 | 8.7 | 9.7 | 9.6 | 10.6 |
| Arginine | 43.9 | 42.3 | 50.6 | 48.1 | 51.0 |
| Non-essential amino acid | |||||
| Taurine | 11.5 | 12.1 | 11.9 | 13.3 | 13.6 |
| Aspartic acid | 32.1 | 32.9 | 33.1 | 32.4 | 33.9 |
| Serine | 13.1 | 14.4 | 14.0 | 13.5 | 14.7 |
| Glutamic acid | 97.2 | 103.0 | 75.5 | 77.0 | 85.6 |
| Glycine | 46.5 | 51.0 | 24.8 | 27.1 | 33.5 |
| Alanine | 19.4 | 19.9 | 19.5 | 18.8 | 20.3 |
| Cystine | 3.5 | 3.9 | 3.2 | 4.2 | 4.4 |
| Tyrosine | 14.1 | 13.7 | 14.5 | 14.2 | 15.7 |
| Gene | Primer Sequence (5′ to 3′) | Product Size (bp) | Accession Number 1 |
|---|---|---|---|
| b0at1 | F: CCGGGACGATCATAAGGGTG R: GTGAGCGGTTGTTGGTTTCC | 144 | XM_035634786.2 |
| b0at2 | F: GGCCGCTATGGAATCGGTTA R: CGGGCTTACGATCACAGTCA | 86 | XM_035642803.2 |
| lat4 | F: CCCACAGATTTCCACCTGGG R: AGGTAGCACCTCGTTTTGGG | 164 | XM_035626052.2 |
| pept1a | F: TGGGCAGTAATCCAGTCACG R: TGCCTTCTTGTGGCTTGGAA | 246 | XM_035604255.1 |
| pept1b | F: CCAGTTCACCTTGGAGCCTT R: GGGAGCCGTCCTGTAAACTC | 116 | XM_035629795.2 |
| bckdha | F: CAACACAGAGCCTTCCGAGT R: TTCCTTGCCTGTCCATCACC | 172 | XM_035623516.2 |
| bckdhb | F: TGAGGGAAGTGGCAAACA R: CACAGACCCTGCTGATGG | 234 | XM_035622988.1 |
| bckdk | F: TACGGCTGACATTAGCGACC R: TGAGCGATTCGTACAGGCAG | 109 | XM_035627139.2 |
| bcat2 | F: CGATGATGTGACGGGGGTAA R: GTCCGCGCTCAGATAACGTA | 84 | XM_035614928.2 |
| mtor | F: CAGGCGGTACATTGGTCCTC R: CTCCACACTTGGGCTACCTG | 110 | XM_035644862.2 |
| s6ka | F: GGCGGACTACGATGCCTTAG R: TGACCTCACTGCTAACGCTG | 76 | XM_035649934.2 |
| s6kb1a | F: CAAGGGAACAGAGCAGAT R: GAGGTCCACAATGAAAGG | 235 | XM_035625497.2 |
| s6kb1b | F: GTGCAGGCTCATCCCTTCTT R: TGGCTGGTGAACTTCGAGTC | 131 | XM_035623426.2 |
| 4e-bp1 | F: CAGACGCCCCAAAATAACGC R: ATCCTCGAGGCTGACTGTCT | 104 | XM_035608367.2 |
| β-actin | F: GTAGGTGATGAAGCCCAGAGCA R: CTGGGTCATCTTCTCCCTGT | 204 | MT023044.1 |
| ef1α | F: TATTAACATCGTGGTCATTGG R: CAGGCGTACTTGAAGGAG | 153 | KU057926.1 |
| Control | Leu-L | Leu-H | Ile-H | Val-H | |
|---|---|---|---|---|---|
| IBW (g) | 21.6 ± 0.1 | 21.5 ± 0.0 | 21.5 ± 0.1 | 21.5 ± 0.1 | 21.5 ± 0.0 |
| FBW (g) | 46.9 ± 1.5 | 42.2 ± 1.5 | 44.4 ± 1.6 | 46.7 ± 1.0 | 45.2 ± 0.9 |
| SR (%) | 98.7 ± 1.3 | 98.7 ± 1.3 | 97.3 ± 2.7 | 100 | 98.7 ± 1.3 |
| WG (%) | 117.8 ± 7.6 | 96.2 ± 7.0 | 106.7 ± 6.7 | 116.5 ± 5.3 | 110.4 ± 4.3 |
| SGR (%) | 1.29 ± 0.06 | 1.12 ± 0.06 | 1.21 ± 0.05 | 1.29 ± 0.04 | 1.24 ± 0.04 |
| FI (%/d) | 1.12 ± 0.02 | 1.10 ± 0.03 | 1.04 ± 0.01 | 1.04 ± 0.03 | 1.04 ± 0.01 |
| FE | 1.11 ± 0.03 ab | 1.00 ± 0.04 b | 1.12 ± 0.03 ab | 1.19 ± 0.05 a | 1.15 ± 0.04 ab |
| PER | 2.17 ± 0.09 ab | 1.93 ± 0.09 b | 2.20 ± 0.06 ab | 2.40 ± 0.10 a | 2.27 ± 0.07 ab |
| PPV (%) | 34.4 ± 1.2 | 32.8 ± 1.1 | 33.4 ± 1.4 | 38.6 ± 2.3 | 34.6 ± 1.1 |
| Control | Leu-L | Leu-H | Ile-H | Val-H | |
|---|---|---|---|---|---|
| Moisture | 774.7 ± 3.2 | 772.0 ± 6.9 | 779.0 ± 2.1 | 775.2 ± 2.6 | 780.8 ± 0.1 |
| Crude protein | 153.0 ± 1.5 | 157.7 ± 4.3 | 148.7 ± 1.0 | 154.1 ± 2.3 | 149.7 ± 0.6 |
| Crude lipid | 26.1 ± 2.8 | 22.3 ± 2.1 | 24.3 ± 1.0 | 23.8 ± 0.4 | 23.0 ± 1.1 |
| Ash | 37.2 ± 1.2 ab | 42.2 ± 1.9 a | 37.5 ± 0.9 ab | 36.7 ± 1.0 b | 36.7 ± 0.2 b |
| Control | Leu-L | Leu-H | Ile-H | Val-H | |
|---|---|---|---|---|---|
| 2 h after feeding | |||||
| Leu | 57.23 ± 4.39 c | 23.73 ± 3.37 d | 225.56 ± 7.58 a | 87.48 ± 5.95 b | 66.38 ± 1.95 bc |
| Ile | 36.40 ± 2.78 b | 47.20 ± 7.55 b | 31.90 ± 6.54 b | 194.57 ± 13.36 a | 46.62 ± 4.90 b |
| Val | 40.85 ± 2.49 b | 48.65 ± 6.13 b | 35.84 ± 5.51 b | 56.67 ± 7.79 b | 218.93 ± 10.10 a |
| 6 h after feeding | |||||
| Leu | 54.19 ± 9.39 b | 22.07 ± 5.24 b | 419.36 ± 50.74 a | 96.66 ± 17.10 b | 87.41 ± 3.64 b |
| Ile | 37.73 ± 7.74 b | 38.26 ± 5.89 b | 61.31 ± 10.03 b | 268.31 ± 16.73 a | 56.32 ± 2.18 b |
| Val | 40.54 ± 6.77 b | 49.35 ± 7.28 b | 60.39 ± 9.34 b | 69.57 ± 16.27 b | 280.91 ± 23.22 a |
| 24 h after feeding | |||||
| Leu | 10.42 ± 0.42 | 11.13 ± 1.83 | 19.34 ± 4.16 | 10.79 ± 0.80 | 13.89 ± 2.84 |
| Ile | 5.64 ± 0.37 | 6.19 ± 1.02 | 5.30 ± 0.73 | 7.02 ± 0.32 | 7.50 ± 1.31 |
| Val | 10.17 ± 0.69 b | 10.56 ± 1.04 b | 9.63 ± 0.88 b | 11.01 ± 0.19 b | 18.47 ± 3.23 a |
| Control | Leu-L | Leu-H | Ile-H | Val-H | |
|---|---|---|---|---|---|
| 2 h after feeding | |||||
| Leu | 740.38 ± 162.82 b | 932.79 ± 122.85 b | 1641.08 ± 87.89 a | 956.04 ± 128.30 b | 935.50 ± 82.50 b |
| Ile | 386.80 ± 78.12 b | 542.40 ± 95.65 b | 511.02 ± 37.59 b | 993.16 ± 57.98 a | 508.65 ± 46.65 b |
| Val | 631.12 ± 143.10 b | 962.63 ± 105.30 ab | 818.41 ± 56.92 ab | 835.97 ± 95.39 ab | 1279.68 ± 92.39 a |
| 6 h after feeding | |||||
| Leu | 646.81 ± 99.77 b | 707.05 ± 74.65 b | 1452.26 ± 151.05 a | 722.37 ± 150.68 b | 708.10 ± 62.01 b |
| Ile | 344.65 ± 62.65 b | 407.99 ± 34.29 b | 460.81 ± 84.68 b | 797.87 ± 85.03 a | 362.69 ± 34.73 b |
| Val | 611.17 ± 78.71 b | 809.13 ± 48.15 ab | 753.11 ± 87.08 ab | 606.43 ± 97.12 b | 985.93 ± 63.40 a |
| 24 h after feeding | |||||
| Leu | 1367.52 ± 127.98 a | 1054.28 ± 132.67 ab | 1270.64 ± 54.57 ab | 882.02 ± 73.18 b | 916.47 ± 95.77 ab |
| Ile | 657.02 ± 52.77 a | 495.51 ± 39.57 ab | 588.33 ± 18.92 ab | 432.07 ± 69.07 b | 441.64 ± 35.35 ab |
| Val | 1163.91 ± 84.20 | 953.66 ± 98.73 | 1086.95 ± 47.05 | 818.78 ± 72.68 | 875.54 ± 73.43 |
| Control | Leu-L | Leu-H | Ile-H | Val-H | |
|---|---|---|---|---|---|
| 2 h after feeding | |||||
| Leu | 144.73 ± 4.69 b | 53.40 ± 20.18 c | 546.82 ± 22.54 a | 186.53 ± 14.14 b | 145.08 ± 9.57 b |
| Ile | 103.55 ± 5.92 b | 112.04 ± 11.20 b | 104.42 ± 4.97 b | 455.06 ± 13.59 a | 116.91 ± 6.95 b |
| Val | 1940.63 ± 116.78 b | 2124.21 ± 142.56 ab | 2050.21 ± 134.59 ab | 2137.86 ± 144.57 ab | 2605.17 ± 75.18 a |
| 6 h after feeding | |||||
| Leu | 169.94 ± 24.60 bc | 69.11 ± 6.75 c | 843.85 ± 32.81 a | 251.48 ± 19.02 b | 195.03 ± 17.17 b |
| Ile | 108.29 ± 14.72 b | 136.45 ± 6.51 b | 136.96 ± 12.41 b | 713.13 ± 25.11 a | 148.39 ± 19.90 b |
| Val | 2219.39 ± 308.48 ab | 2074.76 ± 281.96 ab | 1708.33 ± 229.77 b | 2754.15 ± 185.13 ab | 3098.80 ± 122.82 a |
| 24 h after feeding | |||||
| Leu | 69.30 ± 12.24 | 74.11 ± 4.59 | 114.88 ± 15.84 | 70.53 ± 4.47 | 91.67 ± 6.92 |
| Ile | 48.99 ± 10.82 | 50.36 ± 6.42 | 81.72 ± 14.04 | 78.35 ± 9.69 | 67.39 ± 6.45 |
| Val | 1772.95 ± 92.41 | 1996.23 ± 311.54 | 1705.03 ± 109.53 | 2316.37 ± 258.95 | 2451.37 ± 317.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, J.; Li, Y.; Ma, Q.; Xu, H.; Liang, M.; Wei, Y. Response of Turbot Scophthalmus maximus (Linnaeus, 1758) to Imbalanced Branched-Chain Amino Acids in Diets. Fishes 2023, 8, 534. https://doi.org/10.3390/fishes8110534
Wang L, Liu J, Li Y, Ma Q, Xu H, Liang M, Wei Y. Response of Turbot Scophthalmus maximus (Linnaeus, 1758) to Imbalanced Branched-Chain Amino Acids in Diets. Fishes. 2023; 8(11):534. https://doi.org/10.3390/fishes8110534
Chicago/Turabian StyleWang, Lu, Jinshi Liu, Yanlu Li, Qiang Ma, Houguo Xu, Mengqing Liang, and Yuliang Wei. 2023. "Response of Turbot Scophthalmus maximus (Linnaeus, 1758) to Imbalanced Branched-Chain Amino Acids in Diets" Fishes 8, no. 11: 534. https://doi.org/10.3390/fishes8110534
APA StyleWang, L., Liu, J., Li, Y., Ma, Q., Xu, H., Liang, M., & Wei, Y. (2023). Response of Turbot Scophthalmus maximus (Linnaeus, 1758) to Imbalanced Branched-Chain Amino Acids in Diets. Fishes, 8(11), 534. https://doi.org/10.3390/fishes8110534