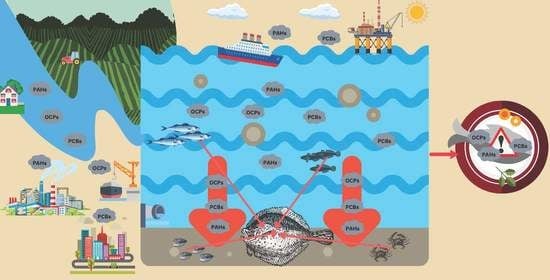
Screening for Organic Pollutants in the Black Sea Turbot (Scophthalmus maeoticus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. PAHs Extraction
2.2. OCP and PCB Extraction
3. Results
3.1. POP Concentrations in the Marine Environment
3.1.1. Water and Sediments
3.1.2. Fish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Nambi, I.M.; Prabhakar Clement, T. Environmental impacts of the Chennai oil spill accident—A case study. Sci. Total Environ. 2018, 626, 795–806. [Google Scholar] [CrossRef]
- Kaufmann, A.; Butcher, P.; Maden, K.; Walker, S.; Widmer, M. Reliability of veterinary drug residue confirmation: High resolution mass spectrometry versus tandem mass spectrometry. Anal. Chim. Acta 2015, 856, 54–67. [Google Scholar] [CrossRef]
- Smith, A.P.; Allen, P.H.; Wadsworth, E.J.K. Navigation Accidents and Their Causes. Navig. Accid. Causes. 2015, pp. 1–14. Available online: www.nautinst.org (accessed on 15 February 2023).
- Aksoy, A.; Das, Y.K.; Yavuz, O.; Guvenc, D.; Atmaca, E.; Agaoglu, S. Organochlorine pesticide and polychlorinated biphenyls levels in fish and mussel in Van Region, Turkey. Bull. Environ. Contam. Toxicol. 2011, 87, 65–69. [Google Scholar] [CrossRef]
- El-Shahawi, M.S.; Hamza, A.; Bashammakh, A.S.; Al-Saggaf, W.T. An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Ross, G. The public health implications of polychlorinated biphenyls (PCBs) in the environment. Ecotoxicol. Environ. Saf. 2004, 59, 275–291. [Google Scholar] [CrossRef]
- Vagi, M.C.; Petsas, A.S.; Kostopoulou, M.N. Potential Effects of Persistent Organic Contaminants on Marine Biota: A Review on Recent Research. Water 2021, 13, 2488. [Google Scholar] [CrossRef]
- De Rosa, E.; Montuori, P.; Triassi, M.; Masucci, A.; Nardone, A. Occurrence and Distribution of Persistent Organic Pollutants (POPs) from Sele River, Southern Italy: Analysis of Polychlorinated Biphenyls and Organochlorine Pesticides in a Water–Sediment System. Toxics 2022, 10, 662. [Google Scholar] [CrossRef]
- Pribylova, P.; Kares, P.; Boruvkova, J.; Cupr, P.; Prokes, R.; Kohoutek, J.; Holoubek, I.; Klanova, J. Levels of persistent organic pollutants and polycyclic aromatic hydrocarbons in ambient air of Central and Eastern Europe. Atmos. Pollut. Res. 2012, 3, 494–505. [Google Scholar] [CrossRef]
- da Silva, A.P.A.; De Oliveira, C.D.L.; Quirino, A.M.S.; Da Silva, F.D.M.; de Aquino Saraiva, R.; Silva-Cavalcanti, J.S. Endocrine Disruptors in Aquatic Environment: Effects and Consequences on the Biodiversity of Fish and Amphibian Species. Aquat. Sci. Technol. 2018, 6, 35. [Google Scholar] [CrossRef]
- Fazio, F.; Saoca, C.; Costa, G.; Zumbo, A.; Piccione, G.; Parrino, V. Flow cytometry and automatic blood cell analysis in striped bass Morone saxatilis (Walbaum, 1792): A new hematological approach. Aquaculture 2019, 513, 734398. [Google Scholar] [CrossRef]
- Basova, M.; Krasheninnikova, S.; Parrino, V. Intra-Decadal (2012–2021) Dynamics of Spatial Ichthyoplankton Distribution in Sevastopol Bay (Black Sea) Affected by Hydrometeorological Factors. Animals 2022, 12, 3317. [Google Scholar] [CrossRef]
- Mulvad, G.; Pedersen, H.S.; Hansen, J.C.; Dewailly, E.; Jul, E.; Pedersen, M.B.; Bjerregaard, P.; Malcom, G.T.; Deguchi, Y.; Middaugh, J.P. Exposure of Greenlandic Inuit to organochlorines and heavy metals through the marine food-chain: An international study. Sci. Total Environ. 1996, 186, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Nag, K. Estimation of hemosiderosis in seabirds and fish exposed to petroleum. Bull. Environ. Contam. Toxicol. 1993, 50, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Barrie, L.A.; Gregor, D.; Hargrave, B.; Lake, R.; Muir, D.; Shearer, R.; Tracey, B.; Bidleman, T. Arctic contaminants: Sources, occurrence and pathways. Sci. Total Environ. 1992, 122, 1–74. [Google Scholar] [CrossRef]
- Lee, J. The regional economic impact of oil and gas extraction in Texas. Energy Policy 2015, 87, 60–71. [Google Scholar] [CrossRef]
- Haritos, N. Introduction to the Analysis and Design of Offshore Structures—An Overview. Electron. J. Struct. Eng. 2007, 7, 3. [Google Scholar] [CrossRef]
- Livingston, M.; Brown, D.; Gabbard, J.; Rosenblum, L.; Baillot, Y.; Julier, S.; Swan, J.; Hix, D. An augmented reality system for military operations in urban terrain. Comput. Graph. 2003, 27, 873–885. [Google Scholar] [CrossRef]
- Fillaudeau, L.; Blanpain-Avet, P.; Daufin, G. Water, wastewater and waste management in brewing industries. J. Clean. Prod. 2006, 14, 463–471. [Google Scholar] [CrossRef]
- Evans, A.E.; Mateo-Sagasta, J.; Qadir, M.; Boelee, E.; Ippolito, A. Agricultural water pollution: Key knowledge gaps and research needs. Curr. Opin. Environ. Sustain. 2019, 36, 20–27. [Google Scholar] [CrossRef]
- Cooper, D.A. HCB, PCB, PCDD and PCDF emissions from ships. Atmos. Environ. 2005, 39, 4901–4912. [Google Scholar] [CrossRef]
- Kumari, K.; Sharma, J.K.; Kanade, G.S.; Kashyap, S.M.; Juwarkar, A.A.; Wate, S.R. Investigation of polybrominated diphenyl ethers in old consumer products in India. Environ. Monit. Assess. 2014, 186, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Leonard, L. Incineration and POPs Release in South Africa. Int. POPs Elimin. Proj. 2005, 5, 2–14. [Google Scholar] [CrossRef]
- Westwood, A. Ocean power. Wave and tidal energy review. Marit. Stud. Manag. 2004, 2, 202–214. [Google Scholar] [CrossRef]
- Pitcher, T.J.; Watson, R.; Forrest, R.; Valtýsson, H.P.; Guénette, S. Estimating illegal and unreported catches from marine ecosystems: A basis for change. Fish Fish. 2002, 3, 317–339. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Review Article. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Sakan, S.; Ostojić, B.; Đorđević, D. Persistent organic pollutants (POPs) in sediments from river and artificial lakes in Serbia. J. Geochem. Explor. 2017, 180, 91–100. [Google Scholar] [CrossRef]
- Dinç, B.; Çelebi, A.; Avaz, G.; Canlı, O.; Güzel, B.; Eren, B.; Yetis, U. Spatial distribution and source identification of persistent organic pollutants in the sediments of the Yeşilırmak River and coastal area in the Black Sea. Mar. Pollut. Bull. 2021, 172, 112884. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Munshi, C. Endocrine Disruptors in Freshwater: Impact on Teleost Reproduction. Proc. Zool. Soc. 2021, 74, 369–377. [Google Scholar] [CrossRef]
- Wolska, L.; Mechlińska, A.; Rogowska, J.; Namieśnik, J. Sources and fate of PAHs and PCBs in the marine environment. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1172–1189. [Google Scholar] [CrossRef]
- Lyons, B.P.; Bignell, J.P.; Stentiford, G.D.; Bolam, T.P.C.; Rumney, H.S.; Bersuder, P.; Barber, J.L.; Askem, C.E.; Nicolaus, M.E.E.; Maes, T. Determining Good Environmental Status under the Marine Strategy Framework Directive: Case study for descriptor 8 (chemical contaminants). Mar. Environ. Res. 2017, 124, 118–129. [Google Scholar] [CrossRef]
- Brasher, A.M.D.; Wolff, R.H. Relations between Land Use and Organochlorine Pesticides, PCBs, and Semi-Volatile Organic Compounds in Streambed Sediment and Fish on the Island of Oahu, Hawaii. Arch. Environ. Contam. Toxicol. 2004, 46, 385–398. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of polycyclic aromatic hydrocarbons for aquatic animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef] [PubMed]
- Timoney, K.P.; Lee, P. Polycyclic aromatic hydrocarbons increase in Athabasca river delta sediment: Temporal trends and environmental correlates. Environ. Sci. Technol. 2011, 45, 4278–4284. [Google Scholar] [CrossRef] [PubMed]
- Chiffre, A.; Degiorgi, F.; Morin-Crini, N.; Bolard, A.; Chanez, E.; Badot, P.M. PAH occurrence in chalk river systems from the Jura region (France). Pertinence of suspended particulate matter and sediment as matrices for river quality monitoring. Environ. Sci. Pollut. Res. 2015, 22, 17486–17498. [Google Scholar] [CrossRef]
- Skupińska, K.; Misiewicz, I.; Kasprzycka-Guttman, T. Polycyclic aromatic hydrocarbons: Physicochemical properties, environmental appearance and impact on living organisms. Acta Pol. Pharm. Drug Res. 2004, 61, 233–240. [Google Scholar]
- World Health Organization, IARC monographs on the evaluation of carcinogenic risks to humans. J. Clin. Pathol. 2010, 93, 9–38.
- Antipa, G.; Marea, N. Oceanografia, Bionomia și Biologia Generală a Mării Negre; Academia Română, Fondul Vasile Adamachi, Monitorul Oficial: Bucharest, Romania, 1941. [Google Scholar]
- Vespremeanu, E.; Golumbeanu, M. The Black Sea: Physical, Environmental and Historical Perspective; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Oros, A.; Lazar, L.; Coatu, V.; Tiganus, D. Recent Data From Pollution Monitoring and Assessment of the Romanian Black Sea Ecosystem, within Implementation of the European Marine Strategy Framework Directive. Water Resour. For. Mar. Ocean Ecosyst. Conf. Proc. SGEM 2016, 2016, 821–828. [Google Scholar] [CrossRef]
- Guerreiro, J. The Blue Growth Challenge to Maritime Governance. Front. Mar. Sci. 2021, 8, 681546. [Google Scholar] [CrossRef]
- Lazar, L.; Oros, A.; Coatu, V.; Boicenco, L.; Timofte, F.; Marin, O.; Vlas, O.; Fiilimon, A.; Galațchi, M.; Maximov, V.; et al. Studiul Privind Evaluarea “Punctelor fierbinți” din Zona Mării Negre, în Conformitate cu Prevederile Protocolului Privind Protecția Mediului Marin al Mării Negre Împotriva Poluării Provenite din Surse și Activități de pe Uscat. 2014; (unpublished). [Google Scholar]
- Baiandna, I.; Giragosov, V.; Khanaychenko, A. Male reproductive potential in the Black Sea turbot (Scophthalmus maximus) spawning populations. Fish. Res. 2022, 253, 106367. [Google Scholar] [CrossRef]
- Niță, V.; Nenciu, M.; Galațchi, M. Speciile de Pești de la Litoralul Românesc. Atlas Actualizat; INCDM: Constanta, Romania, 2022. [Google Scholar]
- Li, X.; Ji, L.; Wu, L.; Gao, X.; Li, X.; Li, J.; Liu, Y. Effect of flow velocity on the growth, stress and immune responses of turbot (Scophthalmus maximus) in recirculating aquaculture systems. Fish Shellfish Immunol. 2019, 86, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- ANPA. Raport de Activitate al Agenției Naționale Pentru Pescuit și Acvacultură; Ministerul Agriculturii şi Dezvoltării Rurale, Agenţia Naţională Pentru Pescuit şi Acvacultura: Bucharest, Romania, 2021; Volume 8, pp. 2505097–2505098. [Google Scholar]
- ANPA. Raport de Activitate al Agenției Naționale Pentru Pescuit și Acvacultură; Ministerul Agriculturii şi Dezvoltării Rurale, Agenţia Naţională Pentru Pescuit şi Acvacultura: Bucharest, Romania, 2022; Volume 8, pp. 2505097–2505098. [Google Scholar]
- The Commission on the Protection of the Black Sea Against Pollution. Available online: https://www.blacksea-commission.org/ (accessed on 7 February 2023).
- Laane, R.W.P.M.; Slijkerman, D.; Vethaak, A.D.; Schobben, J.H.M. Assessment of the environmental status of the coastal and marine aquatic environment in Europe: A plea for adaptive management. Estuarine. Coast. Shelf Sci. 2012, 96, 31–38. [Google Scholar] [CrossRef]
- Directive 2008/56/EU of the Marina Strategy Framework Directive. 2010, pp. 1–52. Available online: https://op.europa.eu/en/publication-detail/-/publication/c6b7a7c8-a550-47f8-8e94-ebe9d9e76853/language-en (accessed on 6 February 2023).
- Dupont, C.; Belin, A.; Moreira, G.; Cochrane, S.; Wilson, L.; Emblow, C.; Kater, B.; Clercs, S.; Des Parr, W.; Le Visage, C.; et al. Article 12 Technical Assessment of the MSFD 2012 Obligations; Milieu Ltd.: Brussels, Belgium, 2014. [Google Scholar]
- Dupont, C.; Belin, A.; Bastiaan, V.; Moreira, G. Article 12 Technical Assessment of the MSFD 2014 Reporting on Monitoring Programmes. Black Sea Regional Report 2014, 20. Available online: https://www.researchgate.net/publication/272350373_Article_12_Technical_Assessment_of_the_MSFD_2012_obligations_reports_for_the_Regional_Seas_-_Black_Sea (accessed on 30 January 2023).
- Boicenco, L.; Abaza, V.; Anton, E.; Bișinicu, E.; Buga, L.; Coatu, V.; Damir, N.; Diaconeasa, D.; Dumitrache, C.; Filimon, A.; et al. Studiu Privind Elaborarea Raportului Privind Starea Ecologică a Ecosistemului Marin Marea Neagră Conform Cerinţelor Art. 17 ale Directivei Cadru Strategia Pentru Mediul Marin (2008/56/EC). 2018, p. 331. Available online: https://cdr.eionet.europa.eu/ro/eu/msfd_art17/2018reporting/textreport/envxzia0w/Romania_roof-report_8a_8b_9_10.pdf (accessed on 23 January 2023).
- Kilemade, M.; Hartl, M.G.J.; O’Halloran, J.; O’Brien, N.M.; Sheehan, D.; Mothersill, C.; van Pelt, F.N.A.M. Effects of contaminated sediment from Cork Harbour, Ireland on the cytochrome P450 system of turbot. Ecotoxicol. Environ. Saf. 2009, 72, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Le Dû-Lacoste, M.; Akcha, F.; Dévier, M.H.; Morin, B.; Burgeot, T.; Budzinski, H. Comparative study of different exposure routes on the biotransformation and genotoxicity of PAHs in the flatfish species, Scophthalmus maximus. Environ. Sci. Pollut. Res. 2013, 20, 690–707. [Google Scholar] [CrossRef]
- Tigănuş, D.; Coatu, V.; Lazăr, L.; Oros, A.; Daiana Spînu, A. Identification of the Sources of Polycyclic Aromatic Hydrocarbons in Sediments from the Romanian Black Sea Sector. Cercet. Mar. Rech. Mar. 2013, 43, 187–196. [Google Scholar]
- Zhuravel, E.V.; Chernyaev, A.P.; Sokolova, L.I.; Chudovskaya, Y.M.; Proshina, M.A. Hydrocarbons and polychlorinated biphenyls in the bottom sediments from the Nakhodka Bay (Peter the Great Bay, Sea of Japan): Assessment of pollution level and potential toxicity. Contemp. Probl. Ecol. 2015, 8, 772–779. [Google Scholar] [CrossRef]
- Coatu, V.; Oros, A.; Ţigănuş, D.; Sht, G.; Bat, L.; Shtereva, G.; Bat, L. Assessment of the Contaminants in Biota From the Western Black Sea Basin in Respect with Msfd Requirements in the Frame of the Misis Project. Rev. Cercet. Mar. Rev. Rech. Mar. Mar. Res. J. 2016, 46, 82–97. [Google Scholar]
- Coatu, V.; Ţigănuş, D.; Oros, A.; Lazăr, L. Analysis of hazardous substance contamination of the marine ecosystem in the Romanian Black Sea coast, part of the Marine Strategy Framework Directive (2008/56/EEC) implementation. Mar. Res. J. 2013, 43, 144–186. [Google Scholar]
- Damir, N.A.; Coatu, V.; Pantea, E.D.; Galațchi, M.; Botez, E.; Birghilă, S. Assessment of Polycyclic Aromatic Hydrocarbons Content in Marine Organisms of Commercial Interest from the Romanian Black Sea Coast. Polycycl. Aromat. Compd. 2022, 42, 7595–7606. [Google Scholar] [CrossRef]
- Coatu, V.; Oros, A.; Damir, N.; Timofte, F.; Lazăr, L. Bioaccumulation of Contaminants in the Main Links of the Pelagic Trophic Chain at the Romanian Black Sea Coast. Mar. Res. J. 2018, 48, 118–134. [Google Scholar]
- Damir, N.; Danilov, D.; Oros, A. Chemical status evaluation of the Romanian Romanian Black Sea marine environment based on benthic organisms’ contamination. Mar. Res. J. 2022, 52, 52–77. [Google Scholar] [CrossRef]
- Stancheva, M.; Georgieva, S.; Makedonski, L. Polychlorinated biphenyls in fish from Black Sea, Bulgaria. Food Control 2017, 72, 205–210. [Google Scholar] [CrossRef]
- Bat, L.; Öztekin, A.; Şahin, F.; Arıcı, E.; Özsandıkçı, U. An overview of the Black Sea pollution in Turkey. Mediterr. Fish. Aquac. Res. 2018, 1, 167–186. [Google Scholar] [CrossRef]
- Bakan, G.; Büyükgüngör, H. The Black Sea. Mar. Pollut. Bull. 2000, 41, 24–43. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Worldwide and Regional Laboratory Comparison on the Determination of Organochlorine Compounds, Polybrominated Diphenyl Ethers and Petroleum Hydrocarbons in IAEA-451 Clam (Gafrarium tumidium) Sample, Vienna. 2013. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/IAEA-AQ-28_web.pdf (accessed on 17 January 2023).
- Gómez-Gutiérrez, A.; Garnacho, E.; Bayona, J.M.; Albaigés, J. Assessment of the Mediterranean sediments contamination by persistent organic pollutants. Environ. Pollut. 2007, 148, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Lao, Q.; Liu, G.; Zhou, X.; Chen, F.; Zhang, S. Sources of polychlorinated biphenyls (PCBs) and dichlorodiphenyltrichloroethanes (DDTs) found in surface sediment from coastal areas of Beibu Gulf: A reflection on shipping activities and coastal industries. Mar. Pollut. Bull. 2021, 167, 112318. [Google Scholar] [CrossRef]
- Stoichev, T.; Makedonski, L.; Trifonova, T.; Stancheva, M.; Ribarova, F. DDT in fish from the Bulgarian region of the Black Sea. Chem. Ecol. 2007, 23, 191–200. [Google Scholar] [CrossRef]
- Stockholm Convention. Available online: https://chm.pops.int/ (accessed on 8 February 2023).
- Stancheva, M.; Makedonski, L.; Georgieva, S. Organochlorine pollutants in bluefish (Pomatomus saltatrix) from Bulgarian Black Sea Coast. Bulg. Sci. Pap. 2010, 37, 125–130. [Google Scholar]
- Stancheva, M.; Georgieva, S.; Makedonski, L. Persistent organic pollutants—PCBs and DDTs in fish from Danube River and from Black Sea, Bulgaria. In Proceedings of the CBU International Conference Proceedings 2013—Integration and Innovation in Science and Education, Prague, Czech Republic, 2 June 2013; pp. 354–361. [Google Scholar] [CrossRef]
- Miguel, A.H.; Pereira, P.A.P. Benzo(k)fluoranthene, benzo(ghi)perylene, and indeno(1,2,3-cd)pyrene: New tracers of automotive emissions in receptor modeling. Aerosol Sci. Technol. 1989, 10, 292–295. [Google Scholar] [CrossRef]
- Snedeker, S.M. Pesticides and breast cancer risk: A review of DDT, DDE and dieldrin. Environ. Health Perspect. 2001, 109 (Suppl. S1), 35–47. [Google Scholar] [CrossRef]
- Todd, S.J.; Cain, R.B.; Schmidt, S. Biotransformation of naphthalene and diaryl ethers by green microalgae. Biodegradation 2002, 13, 229–238. [Google Scholar] [CrossRef]
- Varanasi, U.; Gmur, D.J.; Treseler, P.A. Influence of time and mode of exposure on biotransformation of naphthalene by Juvenile starry flounder (Platichthys stellatus) and rock sole (Lepidopsetta bilineata). Arch. Environ. Contam. Toxicol. 1979, 8, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Lazar, L. (Ed.) ANEMONE Deliverable 2.1. In Anthropogenic Pressures and Impact on the Black Sea Costal Coastal Ecosystem; CD Press: Bucharest, Romania, 2021; p. 167. [Google Scholar]
- Lazar, L. (Ed.) ANEMONE Deliverable 2.1. In Impact of the Rivers on the Black Sea Ecosystem; CD Press: Bucharest, Romania, 2021; p. 225. [Google Scholar]
- Panseri, S.; Chiesa, L.; Ghisleni, G.; Marano, G.; Boracchi, P.; Ranghieri, V.; Malandra, R.M.; Roccabianca, P.; Tecilla, M. Persistent organic pollutants in fish: Biomonitoring and cocktail effect with implications for food safety. Food Addit. Contam. Part A 2019, 36, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Streit, B. Bioaccumulation of Contaminants in fish. Fish Ecotoxicol. 1998, 1, 209–226. [Google Scholar] [CrossRef]
- Burkhard, L.P. Evaluation of Published Bioconcentration Factor (BCF) and Bioaccumulation Factor (BAF) Data for Per- and Polyfluoroalkyl Substances Across Aquatic Species. Environ. Toxicol. Chem. 2021, 40, 1530–1543. [Google Scholar] [CrossRef]
- Arnot, J.A.; Gobas, F.A.P.C. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Kwok, C.K.; Liang, Y.; Leung, S.Y.; Wang, H.; Dong, Y.H.; Young, L.; Giesy, J.P.; Wong, M.H. Biota-sediment accumulation factor (BSAF), bioaccumulation factor (BAF), and contaminant levels in prey fish to indicate the extent of PAHs and OCPs contamination in eggs of waterbirds. Environ. Sci. Pollut. Res. 2013, 20, 8425–8434. [Google Scholar] [CrossRef]
- Massone, C.G.; dos Santos, A.A.; Ferreira, P.G.; Carreira, R. Persistent Organic Pollutants (POPs) in Sardine (Sardinella brasiliensis): Biomonitoring and Potential Human Health Effects. Int. J. Environ. Res. Public Health 2023, 20, 2036. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. DDT, LINDANE, and 2,4-D IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2018, Volume 113. Available online: https://publications.iarc.fr/550 (accessed on 6 March 2023).








| Nr. Crt. | Sampling Station | Collection Date (dd/mm/yyyy) | Bottom Depth (m) | Turbot Sex | Weight (g) | Humidity (%) |
|---|---|---|---|---|---|---|
| 1 | Sf. Gheorghe | 13 May 2021 | 50.5 | F | 1100 | 78.3 |
| 2 | Sf. Gheorghe | 13 May 2021 | 50.5 | F | 2400 | 75.6 |
| 3 | Sf. Gheorghe | 13 May 2021 | 43.3 | F | 6400 | 79.2 |
| 4 | Sf. Gheorghe | 13 May 2021 | 40.5 | F | 5350 | 77.4 |
| 5 | Platforms | 13 April 2021 | 51.1 | F | 4900 | 81.0 |
| 6 | Est Constanta | 13 May 2021 | 40.3 | M | 3650 | 77.2 |
| 7 | Est Constanta | 13 May 2021 | 40.3 | F | 2400 | 76.1 |
| PAHs | Retention Time | m/z | Linearity (R2) | LOD µg/g |
|---|---|---|---|---|
| Naphthalene | 7.25 | 128 | 0.9885 | 0.0001 |
| Acenaphthylene | 13.70 | 152 | 0.9937 | 0.0001 |
| Acenaphthene | 14.50 | 154 | 0.9933 | 0.0001 |
| Fluorene | 16.57 | 166 | 0.9945 | 0.0001 |
| Phenanthrene | 20.36 | 178 | 0.9922 | 0.0001 |
| Anthracene | 20.56 | 178 | 0.9968 | 0.0001 |
| Fluoranthene | 25.04 | 202 | 0.9923 | 0.0001 |
| Pyrene | 25.87 | 202 | 0.9980 | 0.0001 |
| Benzo[a]anthracene | 30.74 | 228 | 0.9972 | 0.0001 |
| Chrysene | 30.90 | 228 | 0.9946 | 0.0001 |
| Benzo[b]fluoranthene | 33.80 | 252 | 0.9840 | 0.0001 |
| Benzo[k]fluoranthene | 34.00 | 252 | 0.9734 | 0.0001 |
| Benzo[a]pyrene | 34.68 | 252 | 0.9858 | 0.0001 |
| Benzo(g,h,i)perylene | 37.20 | 276 | 0.9765 | 0.0001 |
| Dibenzo(a,h)anthracene | 37.40 | 278 | 0.9823 | 0.0001 |
| Indeno(1,2,3-c,d)pyrene | 37.74 | 276 | 0.9756 | 0.0001 |
| OCPs | Retention Time | Linearity (R2) | LOD µg/g |
|---|---|---|---|
| Hexacholorbenzen | 3.176 | 0.9925 | 0.0005 |
| Lindan | 5.118 | 0.9909 | 0.0004 |
| Heptachlor | 7.060 | 0.9909 | 0.0003 |
| Aldrin | 7.886 | 0.9587 | 0.0003 |
| Dieldrin | 11.220 | 0.9860 | 0.0003 |
| Endrin | 12.200 | 0.9945 | 0.0004 |
| p,p’DDE | 10.800 | 0.9751 | 0.0002 |
| p,p’DDD | 12.790 | 0.9917 | 0.0002 |
| p,p’DDT | 15.100 | 0.9909 | 0.0002 |
| PCBs | Retention Time | Linearity (R2) | LOD µg/g |
|---|---|---|---|
| PCB28 | 18.827 | 0.9996 | 0.0004 |
| PCB52 | 20.801 | 0.9979 | 0.0003 |
| PCB101 | 25.880 | 0.9988 | 0.0006 |
| PCB118 | 29.591 | 0.9992 | 0.0004 |
| PCB153 | 30.819 | 0.9994 | 0.0006 |
| PCB138 | 32.362 | 0.9952 | 0.0007 |
| PCB180 | 36.257 | 0.9992 | 0.0003 |
| Nr. Crt. | Sampling Station | Collection Date | Bottom Depth (m) | Associated Pressures |
|---|---|---|---|---|
| 1 | Sf. Gheorghe | 27 May 2021 | 40 | Danube’s input |
| 2 | Platforms | 28 May 2021 | 50 | Danube’s input, offshore activities, shipping and port activities |
| 3 | Est Constanta | 30 May 2021 | 44 | Shipping and port activities Dredging and coastal rehabilitation works |
| Legislation | Compound | OPs Group | Value (µg/g Wet Tissue) | |
|---|---|---|---|---|
| COMMISSION REGULATION (EC) 1881/2006 setting maximum levels for certain contaminants in foodstuffs | Benzo[a]pyrene | PAH | 0.002 | Fish fillets, other than smoked fish |
| Order 147/2004 for the approval of veterinary sanitary and food safety rules regarding pesticide residues in animal and non-animal products and veterinary drug residues in animal products. | Aldrin | OCP | 0.02 | Fish for human consumption (fillets) |
| Dieldrin | 0.2 | |||
| Sum DDD, DDE, DDT | 0.1 | |||
| Endrin | 0.05 | |||
| HCB | 0.02 | |||
| Heptachlor | 0.02 | |||
| Lindan | 0.1 | |||
| COMMISSION REGULATION (EC) 1881/2006 setting maximum levels for certain contaminants in foodstuffs, amended by Regulation 1259/2011 as regards maximum levels of dioxins, dioxin-like PCBs and non dioxin-like PCBs in foodstuffs | Sum PCB28, 52, 101, 138, 153, 180 | PCB | 0.075 | Fish products and fillets of fish |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danilov, D.; Dediu, L.; Damir, N.A.; Coatu, V.; Lazar, L. Screening for Organic Pollutants in the Black Sea Turbot (Scophthalmus maeoticus). Fishes 2023, 8, 265. https://doi.org/10.3390/fishes8050265
Danilov D, Dediu L, Damir NA, Coatu V, Lazar L. Screening for Organic Pollutants in the Black Sea Turbot (Scophthalmus maeoticus). Fishes. 2023; 8(5):265. https://doi.org/10.3390/fishes8050265
Chicago/Turabian StyleDanilov, Diana, Lorena Dediu, Nicoleta Alexandra Damir, Valentina Coatu, and Luminita Lazar. 2023. "Screening for Organic Pollutants in the Black Sea Turbot (Scophthalmus maeoticus)" Fishes 8, no. 5: 265. https://doi.org/10.3390/fishes8050265
APA StyleDanilov, D., Dediu, L., Damir, N. A., Coatu, V., & Lazar, L. (2023). Screening for Organic Pollutants in the Black Sea Turbot (Scophthalmus maeoticus). Fishes, 8(5), 265. https://doi.org/10.3390/fishes8050265