Size-Structured Method Applied to the Brown Crab Fishery Callinectes bellicosus in the Gulf of California
Abstract
:1. Introduction
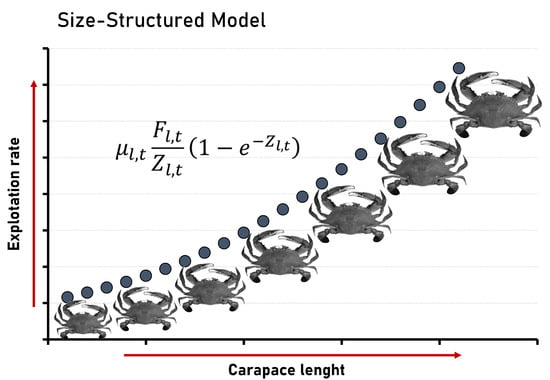
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| 2000 | ||||||
| Females | Males | |||||
| Size (CW) | µl,t | Fl,t | sl | µl,t | Fl,t | sl |
| 82.5 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 |
| 87.5 | 0.000 | 0.000 | 0.008 | 0.000 | 0.000 | 0.002 |
| 92.5 | 0.000 | 0.000 | 0.080 | 0.000 | 0.000 | 0.004 |
| 97.5 | 0.001 | 0.001 | 0.527 | 0.000 | 0.000 | 0.011 |
| 102.5 | 0.001 | 0.001 | 0.945 | 0.000 | 0.000 | 0.029 |
| 107.5 | 0.002 | 0.003 | 0.997 | 0.000 | 0.001 | 0.080 |
| 112.5 | 0.004 | 0.007 | 1.000 | 0.001 | 0.001 | 0.215 |
| 117.5 | 0.020 | 0.034 | 1.000 | 0.001 | 0.002 | 0.484 |
| 122.5 | 0.040 | 0.069 | 1.000 | 0.001 | 0.002 | 0.782 |
| 127.5 | 0.056 | 0.098 | 1.000 | 0.003 | 0.004 | 0.940 |
| 132.5 | 0.064 | 0.114 | 1.000 | 0.008 | 0.014 | 0.987 |
| 137.5 | 0.380 | 1.012 | 1.000 | 0.023 | 0.040 | 0.998 |
| 142.5 | 0.056 | 0.098 | 1.000 | |||
| 147.5 | 0.139 | 0.268 | 1.000 | |||
| 152.5 | 0.444 | 1.325 | 1.000 | |||
| Average | 0.047 | 0.112 | 0.045 | 0.117 | ||
| 2011 | ||||||
| Size (CW) | µl,t | Fl,t | sl | µl,t | Fl,t | sl |
| 87.5 | 0.000 | 0.000 | 0.000 | |||
| 92.5 | 0.001 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 |
| 97.5 | 0.006 | 0.010 | 0.002 | 0.006 | 0.010 | 0.000 |
| 102.5 | 0.032 | 0.055 | 0.047 | 0.031 | 0.054 | 0.012 |
| 107.5 | 0.067 | 0.118 | 0.595 | 0.065 | 0.116 | 0.405 |
| 112.5 | 0.089 | 0.161 | 0.985 | 0.143 | 0.277 | 0.981 |
| 117.5 | 0.149 | 0.292 | 1.000 | 0.244 | 0.535 | 1.000 |
| 122.5 | 0.176 | 0.354 | 1.000 | 0.363 | 0.947 | 1.000 |
| 127.5 | 0.235 | 0.509 | 1.000 | 0.651 | 3.101 | 1.000 |
| 132.5 | 0.444 | 1.325 | 1.000 | 1.149 | 0.000 | 1.000 |
| 137.5 | 0.115 | 0.000 | 1.000 | |||
| 142.5 | ||||||
| 147.5 | ||||||
| Average | 0.119 | 0.257 | 0.295 | 0.560 | ||
| 2014 | ||||||
| Size (CW) | µl,t | Fl,t | sl | µl,t | Fl,t | sl |
| 72.5 | 0.003 | 0.004 | 0.005 | |||
| 77.5 | 0.024 | 0.041 | 0.006 | 0.002 | 0.004 | 0.011 |
| 82.5 | 0.021 | 0.035 | 0.016 | 0.000 | 0.000 | 0.026 |
| 87.5 | 0.036 | 0.062 | 0.044 | 0.019 | 0.033 | 0.066 |
| 92.5 | 0.201 | 0.419 | 0.124 | 0.045 | 0.079 | 0.163 |
| 97.5 | 0.308 | 0.740 | 0.319 | 0.064 | 0.114 | 0.362 |
| 102.5 | 0.423 | 1.216 | 0.631 | 0.073 | 0.131 | 0.639 |
| 107.5 | 0.386 | 1.044 | 0.874 | 0.079 | 0.143 | 0.856 |
| 112.5 | 0.556 | 2.081 | 0.970 | 0.092 | 0.168 | 0.956 |
| 117.5 | 0.639 | 2.932 | 0.994 | 0.067 | 0.120 | 0.989 |
| 122.5 | 0.745 | 4.852 | 0.999 | 0.113 | 0.212 | 0.997 |
| 127.5 | 0.705 | 3.976 | 1.000 | 0.110 | 0.205 | 0.999 |
| 132.5 | 0.679 | 3.504 | 1.000 | 0.196 | 0.406 | 1.000 |
| 137.5 | 0.814 | 0.402 | 1.000 | 0.223 | 0.475 | 1.000 |
| 142.5 | 0.284 | 0.658 | 1.000 | |||
| 147.5 | 0.324 | 0.797 | 1.000 | |||
| 152.5 | 0.343 | 0.866 | 1.000 | |||
| 157.5 | 0.206 | 0.432 | 1.000 | |||
| 162.5 | 0.170 | 0.339 | 1.000 | |||
| 167.5 | 0.045 | 0.079 | 1.000 | |||
| 172.5 | 0.316 | 0.768 | 1.000 | |||
| Average | 0.426 | 1.639 | 0.132 | 0.287 | ||
References
- Ramos-Cruz, S. Estructura y parámetros poblacionales de Callinectes arcuatus Ordway, 1863 (Decapoda: Portunidae), en el sistema lagunar La Joya-Buenavista, Chiapas, México. Pan-Am. J. Aquat. Sci. 2008, 3, 259–268. [Google Scholar]
- Lee, R.F.; Frischer, M.E. The Decline of the Blue Crab. Am. Sci. 2004, 92, 548–553. [Google Scholar] [CrossRef]
- Havens, K.; Bilkovic, D.M.; Stanhope, D.; Angstadt, K. Fishery failure, unemployed commercial fishers, and lost blue crab pots: An unexpected success story. Environ. Sci. Policy 2011, 14, 445–450. [Google Scholar] [CrossRef]
- CONAPESCA. Anuario Estadístico de Acuacultura y Pesca Edición 2013; CONAPESCA: Mazatlán, Mexico, 2013; pp. 1–295. [Google Scholar]
- Heredia-Delgadillo, C.A. Evaluación del Esfuerzo Pesquero Aplicado en la Pesquería de Jaiba Callinectes bellicosus y C. arcuatus en Bahías de Sinaloa. Ph.D. Thesis, Universidad Autónoma de Sinaloa, Mazatlan, Mexico, 2018. [Google Scholar]
- Cisneros-Mata, M.A.; Ramírez-Félix, E.; García-Borbón, J.A.; Castañeda-Fernández de Lara, V.; Labastida-Che, A.; Gómez-Rojo, C.; Madrid-Vera, J. Pesca de Jaiba en el Litoral del Pacífico Mexicano; INAPESCA: Ciudad de Mexico, Mexico, 2014; pp. 1–86. [Google Scholar]
- Munguía-Vega, A.; Torre, J.; Castillo-López, A.; Pfistes, T.; Cudney-Bueno, R. Microsatellite loci for the blue swimming crab (Callinectes bellicosus) (Crustacea: Portunidae) from the Gulf of California, Mexico. Conserv. Genet. Resour. 2010, 2, 135–137. [Google Scholar] [CrossRef]
- Molina-Ocampo, R.E.; Márquez-Farías, J.F.; Ramírez-Félix, E. Jaiba del Golfo de California; SAGARPA-INP: Mazatlán, Mexico, 2006. [Google Scholar]
- Huato-Soberanis, L.; Haro-Garay, M.J.; Ramírez-Félix, E.; López-González, L.C. Estudio Socioeconómico de la Pesquería de Jaiba en Sinaloa y Sonora; Centro de Investigaciones Biológicas del Noroeste, Centro de Estudios para el Desarrollo Rural Sustentable y la Soberanía Alimentaria: La Paz, Mexico, 2006. [Google Scholar]
- Sullivan, P.J.; Lai, H.L.; Gallucci, V.F. A catch-at-length analysis that incorporates a stochastic model of growth. Can. J. Fish. Aquat. Sci. 1990, 47, 184–198. [Google Scholar] [CrossRef]
- Álvarez-Arellano, A.D.; Gaitán-Moran, J. Lagunas costeras y el litoral mexicano. In Lagunas Costeras y el Litoral Mexicano, 1st ed.; De la Lanza-Espino, G., Cáceres-Martínez, C., Eds.; Universidad Autónoma de Baja California Sur: La Paz, Mexico, 1994; pp. 13–74. [Google Scholar]
- Baranov, F.I. On the question of the biological basis of fisheries. Nauchnyi Issledovatelskii Ikhtiologicheskii Institut Izvestiia 1918, 1, 81–128. [Google Scholar]
- Ricker, W.E. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can. 1975, 191, 1–382. [Google Scholar]
- Doubleday, W.G. A least squares approach to analyzing catch at age data. ICNAF Res. Bull. 1976, 12, 69–81. [Google Scholar]
- Hampton, J.; Kleiber, P.; Langley, A.; Hiramatsu, K. Stock Assessment of Yellowfin Tuna in the Western and Central Pacific Ocean; WCPFC–SC1: Noumea, New Caledonia, 2020; pp. 1–76. [Google Scholar]
- von Bertalanffy, L. A quantitative theory of organic growth (Inquiries on growth laws II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Rodríguez-Domínguez, G.; Castillo-Vargasmachuca, S.G.; Ramírez-Pérez, J.S.; Pérez-González, R.; Aragón-Noriega, E.A. Modelos múltiples para determinar el crecimiento de organismos juveniles de jaiba azul Callinectes arcuatus en cautiverio. Cienc. Pesq. 2014, 22, 29–35. [Google Scholar]
- Hogg, R.V.; Craig, A.T. Introduction to Mathematical Statistics; MacMillan: London, UK, 1970; pp. 1–438. [Google Scholar]
- Quinn, T.J.; Deriso, R.B. Quantitative Fish Dynamics; Oxford University Press: Oxford, UK, 1999; pp. 1–542. [Google Scholar]
- Cerdenares-Ladrón De Guevara, G. Biología del pez vela Istiophorus platypterus (Shaw y Nodder, 1792) en el Golfo de Tehuantepec. Ph.D. Thesis, Centro Interdisciplinario de Ciencias Marinas-Instituto Politécnico Nacional, La Paz, Mexico, 2011. [Google Scholar]
- Neter, J.; Kutner, M.H.; Nachtsheim, C.J.; Wasserman, W. Applied Linear Statistical Models; McGraw-Hill: New York, NY, USA, 1996; pp. 1–1408. [Google Scholar]
- Rodríguez-Domínguez, G.; Castillo-Vargasmachuca, S.; Pérez-González, R.; Aragón-Noriega, E.A. Catch-maximum sustainable yield method applied to the crab fishery (Callinectes spp.) in the Gulf of California. J. Shell. Res. 2014, 33, 45–51. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Holt, S.J. On the Dynamics of Exploited Fish Populations, 1st ed.; Chapman and Hall: London, UK, 1957. [Google Scholar]
- Hernández, L.; Arreola-Lizárraga, J.A. Estructura de tallas y crecimiento de los cangrejos Callinectes arcuatus y C. bellicosus (Decapoda: Portunidae) en la laguna costera Las Guásimas, México. Rev.Biol. Trop. 2007, 55, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Ulibarría-Valenzuela, J.J. Aplicación de un Modelo Predictivo a la Pesquería de la Jaiba Café Callinectes bellicosus en la Bahía Santa María de la Reforma. Bachelor’s Thesis, Facultad de Ciencias del Mar, Universidad Autónoma de Sinaloa, Mazatlán, México, 2003. [Google Scholar]
- Sharpe, D.M.T.; Hendry, A.P. Life history change in commercially exploited fish stocks: An analysis of trends across studies. Evol. Appl. 2009, 2, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.F.; Bell, F.H. Biological statistics of the Pacific halibut fishery, 2. Effect of changes in intensity upon total and yield per unit of gear. Rep. Int. Fish. (Pacific Halibut) Comm. 1934, 8, 49. [Google Scholar]
- Policansky, D. Fishing as a cause of evolution in fishes. In The Exploitation of Evolving Resources, 1st ed.; Stokes, T.K., McGlade, J.M., Law, R., Eds.; Springer: Berlin, Germany, 1993; pp. 8–18. [Google Scholar]
- Martell, S.; Froese, R. A simple method for estimating MSY from catch and Resilience. Fish Fish. 2012, 14, 504–514. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Lizárraga, G.G.; Muñoz-Rubí, H.A.; Rodríguez-Domínguez, G.; Pérez-González, R.; Mendívil-Mendoza, J.E.; Aragón-Noriega, E.A. Size-Structured Method Applied to the Brown Crab Fishery Callinectes bellicosus in the Gulf of California. Fishes 2024, 9, 110. https://doi.org/10.3390/fishes9040110
Ortega-Lizárraga GG, Muñoz-Rubí HA, Rodríguez-Domínguez G, Pérez-González R, Mendívil-Mendoza JE, Aragón-Noriega EA. Size-Structured Method Applied to the Brown Crab Fishery Callinectes bellicosus in the Gulf of California. Fishes. 2024; 9(4):110. https://doi.org/10.3390/fishes9040110
Chicago/Turabian StyleOrtega-Lizárraga, Gilberto Genaro, Horacio Alberto Muñoz-Rubí, Guillermo Rodríguez-Domínguez, Raúl Pérez-González, Jaime Edzael Mendívil-Mendoza, and Eugenio Alberto Aragón-Noriega. 2024. "Size-Structured Method Applied to the Brown Crab Fishery Callinectes bellicosus in the Gulf of California" Fishes 9, no. 4: 110. https://doi.org/10.3390/fishes9040110
APA StyleOrtega-Lizárraga, G. G., Muñoz-Rubí, H. A., Rodríguez-Domínguez, G., Pérez-González, R., Mendívil-Mendoza, J. E., & Aragón-Noriega, E. A. (2024). Size-Structured Method Applied to the Brown Crab Fishery Callinectes bellicosus in the Gulf of California. Fishes, 9(4), 110. https://doi.org/10.3390/fishes9040110